Transgene-Free Cynomolgus Monkey iPSCs Generated under Chemically Defined Conditions
- PMID: 38534402
- PMCID: PMC10969578
- DOI: 10.3390/cells13060558
Transgene-Free Cynomolgus Monkey iPSCs Generated under Chemically Defined Conditions
Abstract
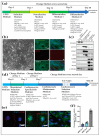
Non-human primates (NHPs) are pivotal animal models for translating novel cell replacement therapies into clinical applications, including validating the safety and efficacy of induced pluripotent stem cell (iPSC)-derived products. Preclinical development and the testing of cell-based therapies ideally comprise xenogeneic (human stem cells into NHPs) and allogenic (NHP stem cells into NHPs) transplantation studies. For the allogeneic approach, it is necessary to generate NHP-iPSCs with generally equivalent quality to the human counterparts that will be used later on in patients. Here, we report the generation and characterization of transgene- and feeder-free cynomolgus monkey (Macaca fascicularis) iPSCs (Cyno-iPSCs). These novel cell lines have been generated according to a previously developed protocol for the generation of rhesus macaque, baboon, and human iPSC lines. Beyond their generation, we demonstrate the potential of the novel Cyno-iPSCs to differentiate into two clinically relevant cell types, i.e., cardiomyocytes and neurons. Overall, we provide a resource of novel iPSCs from the most frequently used NHP species in the regulatory testing of biologics and classical pharmaceutics to expand our panel of iPSC lines from NHP species with high relevance in preclinical testing and translational research.
Keywords: cardiac differentiation; iPSC; macaque; neuronal differentiation; non-human primate; regeneration; stem cell.
Conflict of interest statement
The authors Y.T., N.E., I.R.-P. and R.B., employed by the German Primate Center-Leibniz Institute for Primate Research, and the authors D.R. and E.G.-R., employed by Charles River Laboratories declare no conflict of interest.
Figures


References
-
- Qi Y., Zhang X.-J., Renier N., Wu Z., Atkin T., Sun Z., Ozair M.Z., Tchieu J., Zimmer B., Fattahi F., et al. Combined small-molecule inhibition accelerates the derivation of functional, early-born, cortical neurons from human pluripotent stem cells. Nat. Biotechnol. 2017;35:154–163. doi: 10.1038/nbt.3777. - DOI - PMC - PubMed
-
- Johnsen D.O., Johnson D.K., Whitney R.A. Nonhuman Primates in Biomedical Research. 2nd ed. Elsevier; Amsterdam, The Netherlands: 2012.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

