Engineered Dual Antioxidant Enzyme Complexes Targeting ICAM-1 on Brain Endothelium Reduce Brain Injury-Associated Neuroinflammation
- PMID: 38534474
- PMCID: PMC10968010
- DOI: 10.3390/bioengineering11030200
Engineered Dual Antioxidant Enzyme Complexes Targeting ICAM-1 on Brain Endothelium Reduce Brain Injury-Associated Neuroinflammation
Abstract
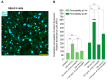
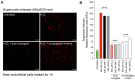
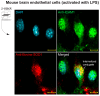
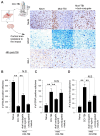
The neuroinflammatory cascade triggered by traumatic brain injury (TBI) represents a clinically important point for therapeutic intervention. Neuroinflammation generates oxidative stress in the form of high-energy reactive oxygen and nitrogen species, which are key mediators of TBI pathology. The role of the blood-brain barrier (BBB) is essential for proper neuronal function and is vulnerable to oxidative stress. Results herein explore the notion that attenuating oxidative stress at the vasculature after TBI may result in improved BBB integrity and neuroprotection. Utilizing amino-chemistry, a biological construct (designated "dual conjugate" for short) was generated by covalently binding two antioxidant enzymes (superoxide dismutase 1 (SOD-1) and catalase (CAT)) to antibodies specific for ICAM-1. Bioengineering of the conjugate preserved its targeting and enzymatic functions, as evaluated by real-time bioenergetic measurements (via the Seahorse-XF platform), in brain endothelial cells exposed to increasing concentrations of hydrogen peroxide or a superoxide anion donor. Results showed that the dual conjugate effectively mitigated the mitochondrial stress due to oxidative damage. Furthermore, dual conjugate administration also improved BBB and endothelial protection under oxidative insult in an in vitro model of TBI utilizing a software-controlled stretching device that induces a 20% in mechanical strain on the endothelial cells. Additionally, the dual conjugate was also effective in reducing indices of neuroinflammation in a controlled cortical impact (CCI)-TBI animal model. Thus, these studies provide proof of concept that targeted dual antioxidant biologicals may offer a means to regulate oxidative stress-associated cellular damage during neurotrauma.
Keywords: blood–brain barrier; brain vasculature; catalase; nanomedicine; neuroinflammation; superoxide dismutase 1; traumatic brain injury.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- Coronado V.G., Xu L., Basavaraju S.V., McGuire L.C., Wald M.M., Faul M.D., Hemphill J.D. Surveillance for traumatic brain injury-related deaths—United States, 1997–2007. MMWR Surveill Summ. 2011;60:1–32. - PubMed
-
- Prevention CfDCa . Surveillance Report of Traumatic Brain Injury-Related Deaths by Age Group, Sex, and Mechanism of Injury—United States, 2018 and 2019. Centers for Disease Control and Prevention, US Department of Health and Human Services; Atlanta, GA, USA: 2022.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

