The Role of Aminopeptidase ERAP1 in Human Pathology-A Review
- PMID: 38534723
- PMCID: PMC10969498
- DOI: 10.3390/cimb46030107
The Role of Aminopeptidase ERAP1 in Human Pathology-A Review
Abstract
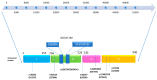
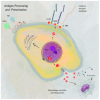
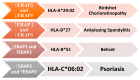
Aminopeptidases are a group of enzymatic proteins crucial for protein digestion, catalyzing the cleavage of amino acids at the N-terminus of peptides. Among them are ERAP1 (coding for endoplasmic reticulum aminopeptidase 1), ERAP2 (coding for endoplasmic reticulum aminopeptidase 2), and LNPEP (coding for leucyl and cystinyl aminopeptidase). These genes encoding these enzymes are contiguous and located on the same chromosome (5q21); they share structural homology and functions and are associated with immune-mediated diseases. These aminopeptidases play a key role in immune pathology by cleaving peptides to optimal sizes for binding to the major histocompatibility complex (MHC) and contribute to cellular homeostasis. By their ability to remove the extracellular region of interleukin 2 and 6 receptors (IL2, IL6) and the tumor necrosis factor receptor (TNF), ERAP1 and ERAP2 are involved in regulating the innate immune response and, finally, in blood pressure control and angiogenesis. The combination of specific genetic variations in these genes has been linked to various conditions, including autoimmune and autoinflammatory diseases and cancer, as well as hematological and dermatological disorders. This literature review aims to primarily explore the impact of ERAP1 polymorphisms on its enzymatic activity and function. Through a systematic examination of the available literature, this review seeks to provide valuable insights into the role of ERAP1 in the pathogenesis of various diseases and its potential implications for targeted therapeutic interventions. Through an exploration of the complex interplay between ERAP1 and various disease states, this review contributes to the synthesis of current biomedical research findings and their implications for personalized medicine.
Keywords: ERAP1; ERAP2; LNEP; aminopeptidases; major histocompatibility complex.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
The Multifaceted Nature of Aminopeptidases ERAP1, ERAP2, and LNPEP: From Evolution to Disease.Front Immunol. 2020 Jul 23;11:1576. doi: 10.3389/fimmu.2020.01576. eCollection 2020. Front Immunol. 2020. PMID: 32793222 Free PMC article.
-
To Be or Not to Be: The Case of Endoplasmic Reticulum Aminopeptidase 2.Front Immunol. 2022 Jun 13;13:902567. doi: 10.3389/fimmu.2022.902567. eCollection 2022. Front Immunol. 2022. PMID: 35769458 Free PMC article. Review.
-
Endoplasmic reticulum aminopeptidases: Biology and pathogenic potential.Nat Rev Rheumatol. 2010 Aug;6(8):461-7. doi: 10.1038/nrrheum.2010.85. Epub 2010 Jun 8. Nat Rev Rheumatol. 2010. PMID: 20531381 Review.
-
The Differential Expression of ERAP1/ERAP2 and Immune Cell Activation in Pre-eclampsia.Front Immunol. 2020 Mar 10;11:396. doi: 10.3389/fimmu.2020.00396. eCollection 2020. Front Immunol. 2020. PMID: 32210971 Free PMC article.
-
Can ERAP1 and ERAP2 Form Functional Heterodimers? A Structural Dynamics Investigation.Front Immunol. 2022 Apr 20;13:863529. doi: 10.3389/fimmu.2022.863529. eCollection 2022. Front Immunol. 2022. PMID: 35514997 Free PMC article.
Cited by
-
Genetic overlap between inflammatory bowel disease and iridocyclitis: insights from a genome-wide association study in a European population.BMC Genom Data. 2024 Oct 29;25(1):92. doi: 10.1186/s12863-024-01274-2. BMC Genom Data. 2024. PMID: 39472800 Free PMC article.
-
Role of endoplasmic reticulum aminopeptidase-1 gene polymorphism (rs13167972) in occurrence susceptibility of ankylosing spondylitis in a sample of Iraqi male patients.Mol Biol Rep. 2024 Mar 29;51(1):462. doi: 10.1007/s11033-024-09438-0. Mol Biol Rep. 2024. PMID: 38551779
-
HLA-B27 and spondyloarthritis: at the crossroads of innate and adaptive immunity.Nat Rev Rheumatol. 2025 Feb;21(2):77-87. doi: 10.1038/s41584-024-01189-3. Epub 2024 Dec 2. Nat Rev Rheumatol. 2025. PMID: 39623156 Review.
References
-
- Wellcome Trust Case Control Consortium. Donnelly P., Barrett J.C., Davison D., Easton D., Evans D.M., Leung H.-T., Marchini J.L., Morris A.P., Spencer C.C.A. Association Scan of 14,500 Nonsynonymous SNPs in Four Diseases Identifies Autoimmunity Variants. Nat. Genet. 2007;39:1329–1337. - PMC - PubMed
-
- Nguyen T.T., Chang S.-C., Evnouchidou I., York I.A., Zikos C., Rock K.L., Goldberg A.L., Stratikos E., Stern L.J. Structural Basis for Antigenic Peptide Precursor Processing by the Endoplasmic Reticulum Aminopeptidase ERAP1. Nat. Struct. Mol. Biol. 2011;18:604–613. doi: 10.1038/nsmb.2021. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

