A Comparison of 68Ga-PSMA PET/CT-Based Split Renal Function with 99mTc-MAG3 Renography in Patients with Metastatic Castration-Resistant Prostate Carcinoma Treated with 177Lu-PSMA
- PMID: 38534999
- PMCID: PMC10968823
- DOI: 10.3390/diagnostics14060578
A Comparison of 68Ga-PSMA PET/CT-Based Split Renal Function with 99mTc-MAG3 Renography in Patients with Metastatic Castration-Resistant Prostate Carcinoma Treated with 177Lu-PSMA
Abstract
Background: Physiological PSMA expression in the cells of the proximal renal tubules and consecutive radiopharmaceutical binding and retention could potentially lead to radioligand-therapy-induced nephrotoxicity. Thus, patients with metastatic castration-resistant prostate cancer undergo 99mTc-Mercaptoacetyltriglycine (MAG3) renal scintigraphy to assess kidney function and to exclude renal obstruction as part of their workup for PSMA-targeted radioligand therapy (RLT). 99mTc-MAG-3 renal scintigraphy often requires an additional visit to the nuclear medicine department and patients spend 30-90 min in the department, which is inconvenient and takes up camera time. In addition, the patients are subjected to a baseline 68Ga-PSMA PET/CT to assess for PSMA-positive disease prior to targeted radioligand therapy. The aim of this retrospective cross-sectional study was to compare 99mTc-MAG-3-based split renal function (SRF) with 68Ga-PSMA-derived SRF.
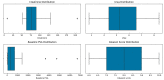
Methods: This retrospective cross-sectional study included 28 patients with histologically proven metastatic castration-resistant prostate cancer (mCRPC) who received 177Lu-PSMA617. A comparison between the split renal function using 68Ga-PSMA PET/CT and the 99mTc-MAG-3-derived split renal function was carried out in 56 kidneys (n = 56). The SRF on 68Ga-PSMA was calculated using the volume and the average standard uptake value (SUVmean) within each VOI calculated as previously described by Roser et al.: SRF = (VOLUMEright) ∗ SUVmeanright/(VOLUMEright ∗ SUVmeanright + VOLUMEleft ∗ SUVmeanleft). Paired tests and correlation coefficients were used to compare 68Ga-PSMA and 99mTc-MAG-3. A visual comparison of kidney morphology on both studies was also performed.
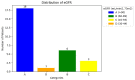
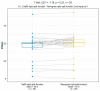
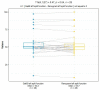
Results: The median SRF of the right kidney was 49.9% (range: 3-91%) using 68Ga-PSMA PET/CT and 50.5% (range: 0-94%) with 99mTc-MAG3 scintigraphy. Notably, there was a strong correlation between SRF measurements obtained from PSMA and 99mTcMAG3, with a Pearson correlation coefficient of 0.957 (p < 0.001). Both 99mTc-MAG3 and 68Ga-PSMA PET/CT studies identified morphological renal abnormalities; there were nine hydronephrotic kidneys, four shrunken kidneys and one obstructed kidney, and there was a strong positive correlation between 68Ga-PSMA kidney morphology and 99mTcMAG3 renal scintigraphy kidney morphology, with a correlation coefficient of 0.93.
Conclusions: PSMA-derived split function demonstrated a high correlation with renal function assessed on diuretic 99mTc-MAG3 renograms. PET-derived split renal function may, therefore, be considered an alternative to diuretic renogram-based split function. Furthermore, both 99mTc-MAG3 and 68Ga-PSMA PET/CT studies identified morphological renal abnormalities such as hydronephrosis, shrunken and obstructed kidneys. This correlation underscores the potential utility of 68Ga-PSMA imaging as a valuable tool for assessing kidney morphology as an alternative to renogram split function in clinical practice.
Keywords: 68Ga PSMA-11; 99mTc-MAG3; PET/CT; kidney; renal function.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- National Collaborating Centre for Cancer (UK) Prostate Cancer: Diagnosis and Treatment. National Collaborating Centre for Cancer; Cardiff, UK: 2008. [(accessed on 1 March 2024)]. Available online: https://www.ncbi.nlm.nih.gov/pubmed/21542543.
-
- Sathekge M., Lengana T., Maes A., Vorster M., Zeevaart J., Lawal I., Ebenhan T., Van de Wiele C. 68Ga-PSMA-11 PET/CT in primary staging of prostate carcinoma: Preliminary results on differences between black and white South-Africans. Eur. J. Nucl. Med. Mol. Imaging. 2018;45:226–234. doi: 10.1007/s00259-017-3852-8. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

