Fusion Imaging of Non-Invasive and Invasive Cardiac Electroanatomic Mapping in Patients with Ventricular Ectopic Beats: A Feasibility Analysis in a Case Series
- PMID: 38535042
- PMCID: PMC10968778
- DOI: 10.3390/diagnostics14060622
Fusion Imaging of Non-Invasive and Invasive Cardiac Electroanatomic Mapping in Patients with Ventricular Ectopic Beats: A Feasibility Analysis in a Case Series
Abstract
In patients with premature ventricular contractions (PVCs), non-invasive mapping could locate the PVCs' origin on a personalized 3-dimensional (3D) heart model and, thus, facilitate catheter ablation therapy planning. The aim of our report is to evaluate its accuracy compared to invasive mapping in terms of assessing the PVCs' early activation zone (EAZ). For this purpose, non-invasive electrocardiographic imaging (ECGI) was performed using the Amycard 01C system (EP Solutions SA, Switzerland) in three cases. In the first step, a multichannel ECG (up to 224 electrodes) was recorded, and the dominant PVCs were registered. Afterward, a cardiac computed tomography (in two cases) or magnetic resonance imaging (in one case) investigation was carried out acquiring non-contrast torso scans for 8-electrode strip visualization and contrast heart acquisition. For the reconstructed epi/endocardial meshes of the heart, non-invasive isochronal maps were generated for the selected multichannel ECG fragments. Then, the patients underwent an invasive electrophysiological study, and the PVCs' activation was evaluated by a 3D mapping system (EnSite NavX Precision, Abbott). Finally, using custom-written software, we performed 3D fusion of the non-invasive and invasive models and compared the resulting isochronal maps. A qualitative analysis in each case showed the same early localization of the dominant PVC on the endocardial surface when comparing the non-invasive and invasive isochronal maps. The distance from the EAZ to the mitral or tricuspid annulus was comparable in the invasive/non-invasive data (36/41 mm in case N1, 73/75 mm in case N2, 9/12 mm in case N3). The area of EAZ was also similar between the invasive/non-invasive maps (4.3/4.5 cm2 in case N1, 7.1/7.0 cm2 in case N2, 0.4/0.6 cm2 in case N3). The distances from the non-invasive to invasive earliest activation site were 4 mm in case N1, 7 mm in case N2, and 4 mm in case N3. Such results were appropriate to trust the clinical value of the preoperative data in these cases. In conclusion, the non-invasive identification of PVCs before an invasive electrophysiological study can guide clinical and interventional decisions, demonstrating appropriate accuracy in the estimation of focus origin.
Keywords: electroanatomic mapping; fusion imaging; non-invasive electrocardiographic imaging; ventricular ectopic beats.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






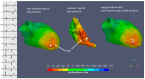

References
-
- Tanner H., Hindricks G., Schirdewahn P., Kobza R., Dorszewski A., Piorkowski C., Gerds-Li J.-H., Kottkamp H. Outflow tract tachycardia with R/S transition in lead V3: Six different anatomic approaches for successful ablation. J. Am. Coll. Cardiol. 2005;45:418–423. doi: 10.1016/j.jacc.2004.10.037. - DOI - PubMed
-
- Chen J., Hoff P.I., Rossvoll O., De Bortoli A., Solheim E., Sun L., Schuster P., Larsen T., Ohm O.-J. Ventricular arrhythmias originating from the aortomitral continuity: An uncommon variant of left ventricular outflow tract tachycardia. Europace. 2011;14:388–395. doi: 10.1093/europace/eur318. - DOI - PubMed
-
- Intini A., Goldstein R.N., Jia P., Ramanathan C., Ryu K., Giannattasio B., Gilkeson R., Stambler B.S., Brugada P., Stevenson W.G., et al. Electrocardiographic imaging (ECGI), a novel diagnostic modality used for mapping of focal left ventricular tachycardia in a young athlete. Heart Rhythm. 2005;2:1250–1252. doi: 10.1016/j.hrthm.2005.08.019. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

