Cardiac Allograft Vasculopathy: Challenges and Advances in Invasive and Non-Invasive Diagnostic Modalities
- PMID: 38535118
- PMCID: PMC10971179
- DOI: 10.3390/jcdd11030095
Cardiac Allograft Vasculopathy: Challenges and Advances in Invasive and Non-Invasive Diagnostic Modalities
Abstract
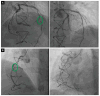
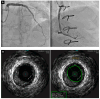
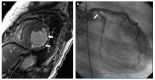
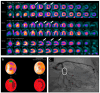
Cardiac allograft vasculopathy (CAV) is a distinct form of coronary artery disease that represents a major cause of death beyond the first year after heart transplantation. The pathophysiology of CAV is still not completely elucidated; it involves progressive circumferential wall thickening of both the epicardial and intramyocardial coronary arteries. Coronary angiography is still considered the gold-standard test for the diagnosis of CAV, and intravascular ultrasound (IVUS) can detect early intimal thickening with improved sensitivity. However, these tests are invasive and are unable to visualize and evaluate coronary microcirculation. Increasing evidence for non-invasive surveillance techniques assessing both epicardial and microvascular components of CAV may help improve early detection. These include computed tomography coronary angiography (CTCA), single-photon emission computed tomography (SPECT), positron emission tomography (PET), and vasodilator stress myocardial contrast echocardiography perfusion imaging. This review summarizes the current state of diagnostic modalities and their utility and prognostic value for CAV and also evaluates emerging tools that may improve the early detection of this complex disease.
Keywords: angiography; cardiac allograft vasculopathy; echocardiography; nuclear imaging.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Sciaccaluga C., Ghionzoli N., Mandoli G.E., Sisti N., D’Ascenzi F., Focardi M., Bernazzali S., Vergaro G., Emdin M., Valente S., et al. The role of non-invasive imaging modalities in cardiac allograft vasculopathy: An updated focus on current evidences. Heart Fail. Rev. 2022;27:1235–1246. doi: 10.1007/s10741-021-10155-0. - DOI - PMC - PubMed
-
- Khush K.K., Cherikh W.S., Chambers D.C., Harhay M.O., Hayes D., Jr., Hsich E., Meiser B., Potena L., Robinson A., Rossano J.W., et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult heart transplantation report—2019; focus theme: Donor and recipient size match. J. Heart Lung Transplant. 2019;38:1056–1066. doi: 10.1016/j.healun.2019.08.004. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

