Toxicological Evaluation toward Refined Montmorillonite with Human Colon Associated Cells and Human Skin Associated Cells
- PMID: 38535268
- PMCID: PMC10971592
- DOI: 10.3390/jfb15030075
Toxicological Evaluation toward Refined Montmorillonite with Human Colon Associated Cells and Human Skin Associated Cells
Abstract
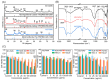
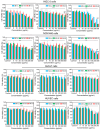
Montmorillonite has been refined to overcome uncertainties originating from different sources, which offers opportunities for addressing various health issues, e.g., cosmetics, wound dressings, and antidiarrheal medicines. Herein, three commercial montmorillonite samples were obtained from different sources and labeled M1, M2, and M3 for Ca-montmorillonite, magnesium-enriched Ca-montmorillonite, and silicon-enriched Na-montmorillonite, respectively. Commercial montmorillonite was refined via ultrasonic scission-differential centrifugation and labeled S, M, or L according to the particle sizes (small, medium, or large, respectively). The size distribution decreased from 2000 nm to 250 nm with increasing centrifugation rates from 3000 rpm to 12,000 rpm. Toxicological evaluations with human colon-associated cells and human skin-associated cells indicated that side effects were correlated with excess dosages and silica sand. These side effects were more obvious with human colon-associated cells. The microscopic interactions between micro/nanosized montmorillonite and human colon-associated cells or human skin-associated cells indicated that those interactions were correlated with the size distributions. The interactions of the M1 series with the human cells were attributed to size effects because montmorillonite with a broad size distribution was stored in the M1 series. The M2 series interactions with human cells did not seem to be correlated with size effects because large montmorillonite particles were retained after refining. The M3 series interactions with human cells were attributed to size effects because small montmorillonite particles were retained after refining. This illustrates that toxicological evaluations with refined montmorillonite must be performed in accordance with clinical medical practices.
Keywords: clinical medical orientation; microscopic interaction; montmorillonite; refine; toxicological evaluation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Polyvinylpyrrolidone adsorption and structural studies on homoionic Li-, Na-, K-, and Cs-montmorillonite colloidal suspensions.J Colloid Interface Sci. 2002 Aug 1;252(1):93-101. doi: 10.1006/jcis.2002.8422. J Colloid Interface Sci. 2002. PMID: 16290767
-
Effect of silica sand on activation energy for diffusion of sodium ions in montmorillonite and silica sand mixture.J Contam Hydrol. 2003 Mar;61(1-4):85-93. doi: 10.1016/S0169-7722(02)00115-8. J Contam Hydrol. 2003. PMID: 12598096
-
Final report on the safety assessment of aluminum silicate, calcium silicate, magnesium aluminum silicate, magnesium silicate, magnesium trisilicate, sodium magnesium silicate, zirconium silicate, attapulgite, bentonite, Fuller's earth, hectorite, kaolin, lithium magnesium silicate, lithium magnesium sodium silicate, montmorillonite, pyrophyllite, and zeolite.Int J Toxicol. 2003;22 Suppl 1:37-102. Int J Toxicol. 2003. PMID: 12851164 Review.
-
Study of individual Na-montmorillonite particles size, morphology, and apparent charge.J Colloid Interface Sci. 2005 May 15;285(2):719-30. doi: 10.1016/j.jcis.2004.12.016. J Colloid Interface Sci. 2005. PMID: 15837491
-
Total allowable concentrations of monomeric inorganic aluminum and hydrated aluminum silicates in drinking water.Crit Rev Toxicol. 2012 May;42(5):358-442. doi: 10.3109/10408444.2012.674101. Crit Rev Toxicol. 2012. PMID: 22512666 Review.
Cited by
-
Clays and Wound Healing.Materials (Basel). 2024 Apr 7;17(7):1691. doi: 10.3390/ma17071691. Materials (Basel). 2024. PMID: 38612205 Free PMC article. Review.
-
Eugenol@Montmorillonite vs. Citral@Montmorillonite Nanohybrids for Gelatin-Based Extruded, Edible, High Oxygen Barrier, Active Packaging Films.Polymers (Basel). 2025 May 29;17(11):1518. doi: 10.3390/polym17111518. Polymers (Basel). 2025. PMID: 40508761 Free PMC article.
References
-
- Murugesan S., Scheibel T. Copolymer/clay nanocomposites for biomedical applications. Adv. Funct. Mater. 2020;30:1908101. doi: 10.1002/adfm.201908101. - DOI
-
- Liu J., Cai W., Khatoon N., Yu W., Zhou C. On how montmorillonite as an ingredient in animal feed functions. Appl. Clay Sci. 2021;202:105963. doi: 10.1016/j.clay.2020.105963. - DOI
-
- Khachani M., Stealey S., Dharmesh E., Kader M., Buckner S., Jelliss P., Zustiak S. Silicate clay-hydrogel nanoscale composites for sustained delivery of small molecules. ACS Appl. Nano Mater. 2022;5:18940–18954. doi: 10.1021/acsanm.2c04721. - DOI
-
- Zhang J., Pan Y., Dong S., Yang M., Huang Z., Yan C., Gao Y. Montmorillonite/agarose three-dimensional network gel sponge for wound healing with hemostatic and durable antibacterial properties. ACS Appl. Nano Mater. 2023;6:17263–17275. doi: 10.1021/acsanm.3c03708. - DOI
-
- Rong R., Xu X., Zhu S., Li B., Wang X., Tang K. Facile preparation of homogeneous and length controllable halloysite nanotubes by ultrasonic scission and uniform viscosity centrifugation. Chem. Eng. J. 2016;291:20–29. doi: 10.1016/j.cej.2016.01.082. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

