Effect of NiO Addition on the Sintering and Electrochemical Properties of BaCe0.55Zr0.35Y0.1O3-δ Proton-Conducting Ceramic Electrolyte
- PMID: 38535280
- PMCID: PMC10972099
- DOI: 10.3390/membranes14030061
Effect of NiO Addition on the Sintering and Electrochemical Properties of BaCe0.55Zr0.35Y0.1O3-δ Proton-Conducting Ceramic Electrolyte
Abstract
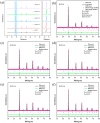
Proton ceramic fuel cells offer numerous advantages compared with conventional fuel cells. However, the practical implementation of these cells is hindered by the poor sintering activity of the electrolyte. Despite extensive research efforts to improve the sintering activity of BCZY, the systematic exploration of the utilization of NiO as a sintering additive remains insufficient. In this study, we developed a novel BaCe0.55Zr0.35Y0.1O3-δ (BCZY) electrolyte and systematically investigated the impact of adding different amounts of NiO on the sintering activity and electrochemical performance of BCZY. XRD results demonstrate that pure-phase BCZY can be obtained by sintering the material synthesized via solid-state reaction at 1400 °C for 10 h. SEM analysis revealed that the addition of NiO has positive effects on the densification and grain growth of BCZY, while significantly reducing the sintering temperature required for densification. Nearly fully densified BCZY ceramics can be obtained by adding 0.5 wt.% NiO and annealing at 1350 °C for 5 h. The addition of NiO exhibits positive effects on the densification and grain growth of BCZY, significantly reducing the sintering temperature required for densification. An anode-supported full cell using BCZY with 0.5 wt.% NiO as the electrolyte reveals a maximum power density of 690 mW cm-2 and an ohmic resistance of 0.189 Ω cm2 at 650 °C. Within 100 h of long-term testing, the recorded current density remained relatively stable, demonstrating excellent electrochemical performance.
Keywords: BaCe0.55Zr0.35Y0.1O3-δ electrolyte; NiO sintering additive; electrochemical performance; proton ceramic fuel cells; sintering activity.
Conflict of interest statement
The authors declare that they have no conflicts of interest here.
Figures






References
-
- Cao J., Ji Y., Shao Z. Perovskites for protonic ceramic fuel cells: A review. Energy Environ. Sci. 2022;15:2200–2232. doi: 10.1039/D2EE00132B. - DOI
-
- Choi S., Kucharczyk C.J., Liang Y., Zhang X., Takeuchi I., Ji H.-I., Haile S.M. Exceptional power density and stability at intermediate temperatures in protonic ceramic fuel cells. Nat. Energy. 2018;3:202–210. doi: 10.1038/s41560-017-0085-9. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

