A Phase Three Study of the Safety and Immunogenicity of a Four-dose Series of 20-Valent Pneumococcal Conjugate Vaccine in Healthy Infants
- PMID: 38535409
- PMCID: PMC11090512
- DOI: 10.1097/INF.0000000000004334
A Phase Three Study of the Safety and Immunogenicity of a Four-dose Series of 20-Valent Pneumococcal Conjugate Vaccine in Healthy Infants
Abstract
Background: The 20-valent pneumococcal conjugate vaccine (PCV20) was developed to extend pneumococcal disease protection beyond 13-valent PCV (PCV13).
Methods: This phase 3, double-blind study conducted in the United States/Puerto Rico evaluated PCV20 safety and immunogenicity. Healthy infants were randomized to receive a 4-dose series of PCV20 or PCV13 at 2, 4, 6 and 12-15 months old. Objectives included demonstrating noninferiority (NI) of PCV20 to PCV13 immunoglobulin G (IgG) geometric mean concentrations after doses 3 and 4 and percentages of participants with predefined IgG concentrations after dose 3, with 7 additional PCV20 serotypes compared with the lowest result among vaccine serotypes in the PCV13 group. Safety assessments included local reactions, systemic events, adverse events, serious adverse events and newly diagnosed chronic medical conditions.
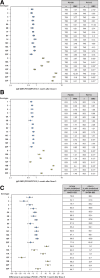
Results: Overall, 1991 participants were vaccinated (PCV20, n = 1001; PCV13, n = 990). For IgG geometric mean concentrations 1 month after both doses 3 and 4, all 20 serotypes met NI criteria (geometric mean ratio lower 2-sided 95% confidence interval > 0.5). For percentages of participants with predefined IgG concentrations after dose 3, NI (percentage differences lower 2-sided 95% confidence interval > -10%) was met for 8/13 matched serotypes and 6/7 additional serotypes; 4 serotypes missed the statistical NI criterion by small margins. PCV20 also elicited functional and boosting responses to all 20 serotypes. The safety profile of PCV20 was similar to PCV13.
Conclusion: A 4-dose series of PVC20 was well tolerated and elicited robust serotype-specific immune responses expected to help protect infants and young children against pneumococcal disease due to the 20 vaccine serotypes. Clinical trial registration: NCT04382326.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Figures



References
-
- Pilishvili T, Lexau C, Farley MM, et al. ; Active Bacterial Core Surveillance/Emerging Infections Program Network. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32–41. - PubMed
-
- World Health Organization. Pneumococcal Conjugate Vaccines in Infants and Children Under 5 Years of Age: WHO Position Paper. February 2019. Available at: https://iris.who.int/bitstream/handle/10665/310968/WER9408.pdf?ua=1. Accessed January 24, 2024.
-
- Centers for Disease Control and Prevention. Manual for the Surveillance of Vaccine-Preventable Diseases. Chapter 11: Pneumococcal. Available at: http://www.cdc.gov/vaccines/pubs/surv-manual/chpt11-pneumo.html. Accessed February 1, 2021.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

