Hydroxytakakiamide and Other Constituents from a Marine Sponge-Associated Fungus Aspergillus fischeri MMERU23, and Antinociceptive Activity of Ergosterol Acetate, Acetylaszonalenin and Helvolic Acid
- PMID: 38535438
- PMCID: PMC10971494
- DOI: 10.3390/md22030097
Hydroxytakakiamide and Other Constituents from a Marine Sponge-Associated Fungus Aspergillus fischeri MMERU23, and Antinociceptive Activity of Ergosterol Acetate, Acetylaszonalenin and Helvolic Acid
Abstract
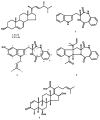
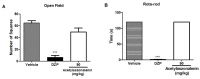
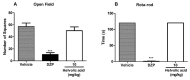
An unreported prenylated indole derivative hydroxytakakiamide (4) was isolated, together with the previously described ergosterol (1), ergosterol acetate (2), and (3R)-3-(1H-indol-3-ylmethyl)-3, 4-dihydro-1H-1,4-benzodiazepine-2,5-dione (3), from the column fractions of the crude ethyl acetate extract of the culture of a marine sponge-associated fungus, Aspergillus fischeri MMERU 23. The structure of 4 was elucidated by the interpretation of 1D and 2D NMR spectral data and high-resolution mass spectrum. The absolute configuration of the stereogenic carbon in 3 was proposed to be the same as those of the co-occurring congeners on the basis of their biogenetic consideration and was supported by the comparison of its sign of optical rotation with those of its steroisomers. The crude ethyl acetate extract and 2 were evaluated, together with acetylaszonalenin (5) and helvolic acid (6), which were previously isolated from the same extract, for the in vivo antinociceptive activity in the mice model. The crude ethyl acetate extract exhibited antinociceptive activity in the acetic acid-induced writhing and formalin tests, while 2, 5, and 6 displayed the effects in the late phase of the formalin test. On the other hand, neither the crude ethyl acetate extract nor 2, 5, and 6 affected the motor performance of mice in both open-field and rotarod tests. Additionally, docking studies of 2, 5, and 6 were performed with 5-lipoxygenase (5-LOX) and phosphodiesterase (PDE) enzymes, PDE4 and PDE7, which are directly related to pain and inflammatory processes. Molecular docking showed that 6 has low affinity energy to PDE4 and PDE7 targets while retaining high affinity to 5-LOX. On the other hand, while 2 did not display any hydrogen bond interactions in any of its complexes, it achieved overall better energy values than 6 on the three antinociceptive targets. On the other hand, 5 has the best energy profile of all the docked compounds and was able to reproduce the crystallographic interactions of the 5-LOX complex.
Keywords: Aspergillaceae; Aspergillus fischeri; acetylaszonalenin; antinociceptive activity; ergosterol acetate; helvolic acid; hydroxytakakiamide; marine sponge-associated fungus.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









References
-
- Cardona H.R.A., Froes T.Q., Souza B.C., Leite F.H.A., Brandão H.N., Buaruang J., Kijjoa A., Alves C.Q. Thermal shift assays of marine-derived fungalmetabolites from Aspergillus fischeri MMERU 23against Leishmania major pteridine reductase 1 and molecular dynamics studies. J. Biomol. Struct. 2022;40:11968–11976. doi: 10.1080/07391102.2021.1966510. - DOI - PubMed
-
- Matta C.B.B., Souza E.T., Queiroz A.C., Lira D.P., Araújo M.V., Cavalcante-Silva L.H.A., Miranda G.E.C., Araújo-Júnior J.X., Barbosa-Filho J.M., Santos B.V.O., et al. Antinociceptive and anti-Inflammatory Activity from algae of the genus Caulerpa. Mar. Drugs. 2011;9:307–318. doi: 10.3390/md9030307. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

