Human Papillomavirus (HPV) Infection and Risk Behavior in Vaccinated and Non-Vaccinated Paraguayan Young Women
- PMID: 38535552
- PMCID: PMC10974315
- DOI: 10.3390/pathogens13030209
Human Papillomavirus (HPV) Infection and Risk Behavior in Vaccinated and Non-Vaccinated Paraguayan Young Women
Abstract
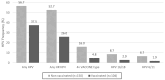
Cervical cancer is a global health concern and ranks fourth among the most prevalent cancers in women worldwide. Human papillomavirus (HPV) infection is a known precursor of cervical cancer and preventive measures include prophylactic vaccines. This study focused on sexually active Paraguayan women aged 18-25 years, exploring the intersection of HPV vaccination and sexual behavior. Among 254 participants, 40.9% received the Gardasil-4 vaccine, with no significant differences in sexual behavior between the vaccinated and unvaccinated sexually active groups. However, a notable decrease in the prevalence of HPV among the vaccinated women highlights the efficacy of this vaccine in reducing infections. The prevalence of any HPV type was 37.5% in vaccinated participants compared to 56.7% in unvaccinated participants (p = 0.0026). High-risk HPV types showed a significant difference, with a prevalence of 26.0% in vaccinated women compared with 52.7% in unvaccinated women (p < 0.001). Although a potential decline in genital warts was observed among the vaccinated individuals, statistical significance (p = 0.0564) was not reached. Despite the challenges in achieving high vaccination coverage, the observed reduction in HPV prevalence underscores the importance of ongoing monitoring, healthcare professional recommendations, and comprehensive risk management. These findings contribute to dispelling concerns about HPV vaccination influencing sexual behavior, advocating further large-scale research to explore the impact of vaccines on various HPV types and potential cross-protection.
Keywords: HPV vaccination; Paraguay; cervical cancer; human papillomavirus; sexual behavior; vaccine impact.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the study design; collection, analyses, or interpretation of data; writing of the manuscript; or decision to publish the results.
Figures
References
-
- Ferlay J., Ervik M., Lam F., Colombet M., Mery L., Piñeros M., Znaor A., Soerjomataram I., Bray F. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. 2020. [(accessed on 20 October 2023)]. Available online: https://gco.iarc.fr/today.
-
- World Health Organization . WHO Position Paper. Volume 97. World Health Organization; Geneva, Switzerland: 2022. Human Papillomavirus Vaccines; pp. 645–672.
-
- Villa L.L., Costa R.L., Petta C.A., Andrade R.P., Ault K.A., Giuliano A.R., Wheeler C.M., Koutsky L.A., Malm C., Lehtinen M., et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: A randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6:271–278. doi: 10.1016/S1470-2045(05)70101-7. - DOI - PubMed
-
- Harper D.M., Franco E.L., Wheeler C.M., Moscicki A.B., Romanowski B., Roteli-Martins C.M., Jenkins D., Schuind A., Costa Clemens S.A., Dubin G., et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: Follow-up from a randomised control trial. Lancet. 2006;367:1247–1255. doi: 10.1016/S0140-6736(06)68439-0. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

