Pd-Based Bimetallic Electrocatalysts for Hydrogen Oxidation Reaction in 0.1 M KOH Solution
- PMID: 38535648
- PMCID: PMC10974835
- DOI: 10.3390/nano14060500
Pd-Based Bimetallic Electrocatalysts for Hydrogen Oxidation Reaction in 0.1 M KOH Solution
Abstract
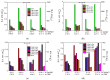
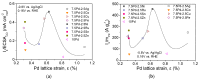
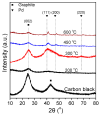
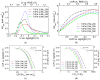
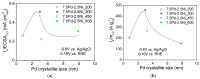
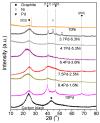
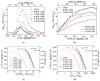
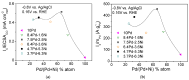
A series of carbon black-supported 7.5 wt.% Pd-2.5 wt.% M/C (M: Ag, Ca, Co, Cu, Fe, Ni, Ru, Sn, Zn) electrocatalysts, synthesized via the wet impregnation method, and reduced at 300 °C, were compared in terms of their hydrogen oxidation reaction (HOR) activity in a 0.1 M KOH solution using the thin-film rotating-disk electrode technique. Moreover, 10 wt.% Pd/C and 10 wt.% Pt/C electrocatalysts were prepared in the same manner and used as references. The 7.5 wt.% Pd-2.5 wt.% Ni/C electrocatalyst exhibited the highest HOR activity among the Pd-based electrocatalysts, although it was lower than that of the 10 wt.% Pt/C. Its activity was also found to be higher than that of Pd-Ni electrocatalysts of the same total metal loading (10 wt.%) and reduction temperature (300 °C) but of different Pd to Ni atomic ratio. It was also higher than that of 7.5 wt.% Pd-2.5 wt.% Ni/C electrocatalysts that were reduced at temperatures other than 300 °C. The superior activity of this electrocatalyst was attributed to an optimum value of the hydrogen binding energy of Pd, which was induced by the presence of Ni (electronic effect), as well as to the oxophilic character of Ni, which favors adsorption on the Ni surface of hydroxyl species that readily react with adsorbed hydrogen atoms on neighboring Pd sites in the rate-determining step.
Keywords: Pd-based electrocatalysts; alkaline medium; hydrogen binding energy; hydrogen oxidation reaction; hydroxyl species binding energy; oxophilic effect; rotating-disk electrode; strain effect.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Electrocatalyst of PdNi Particles on Carbon Black for Hydrogen Oxidation Reaction in Alkaline Membrane Fuel Cell.Nanomaterials (Basel). 2025 Apr 27;15(9):664. doi: 10.3390/nano15090664. Nanomaterials (Basel). 2025. PMID: 40358281 Free PMC article.
-
The Roles of Surface Hydrogen and Hydroxyl in Alkaline Hydrogen Oxidation on Ni-Based Electrocatalysts.ChemSusChem. 2025 Apr 14;18(8):e202402150. doi: 10.1002/cssc.202402150. Epub 2024 Dec 19. ChemSusChem. 2025. PMID: 39648150
-
Isolated Ni Atoms Dispersed on Ru Nanosheets: High-Performance Electrocatalysts toward Hydrogen Oxidation Reaction.Nano Lett. 2020 May 13;20(5):3442-3448. doi: 10.1021/acs.nanolett.0c00364. Epub 2020 Apr 27. Nano Lett. 2020. PMID: 32324412
-
Platinum-based oxygen reduction electrocatalysts.Acc Chem Res. 2013 Aug 20;46(8):1848-57. doi: 10.1021/ar300359w. Epub 2013 Jun 28. Acc Chem Res. 2013. PMID: 23808919 Review.
-
General π-Electron-Assisted Strategy for Ir, Pt, Ru, Pd, Fe, Ni Single-Atom Electrocatalysts with Bifunctional Active Sites for Highly Efficient Water Splitting.Angew Chem Int Ed Engl. 2019 Aug 19;58(34):11868-11873. doi: 10.1002/anie.201904614. Epub 2019 Jul 10. Angew Chem Int Ed Engl. 2019. PMID: 31173428 Review.
References
-
- Zheng J., Zhou S., Gu S., Xu B., Yan Y. Size-Dependent Hydrogen Oxidation and Evolution Activities on Supported Palladium Nanoparticles in Acid and Base. J. Electrochem. Soc. 2016;163:F499–F506. doi: 10.1149/2.0661606jes. - DOI
-
- Gasteiger H.A., Kocha S.S., Sompalli B., Wagner F.T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B Environ. 2005;56:9–35. doi: 10.1016/j.apcatb.2004.06.021. - DOI
-
- Sheng W., Gasteiger H.A., Shao-Horn Y. Hydrogen Oxidation and Evolution Reaction Kinetics on Platinum: Acid vs Alkaline Electrolytes. J. Electrochem. Soc. 2010;157:B1529–B1536. doi: 10.1149/1.3483106. - DOI
-
- Stacy J., Regmi Y.N., Leonard B., Fan M. The recent progress and future of oxygen reduction reaction catalysis: A review. Renew. Sustain. Energy Rev. 2017;69:401–414. doi: 10.1016/j.rser.2016.09.135. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

