Bee Venom: Composition and Anticancer Properties
- PMID: 38535786
- PMCID: PMC10975291
- DOI: 10.3390/toxins16030117
Bee Venom: Composition and Anticancer Properties
Abstract
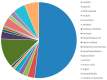
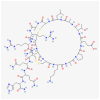
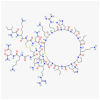
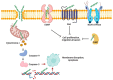
Among the various natural compounds used in alternative and Oriental medicine, toxins isolated from different organisms have had their application for many years, and Apis mellifera venom has been studied the most extensively. Numerous studies dealing with the positive assets of bee venom (BV) indicated its beneficial properties. The usage of bee products to prevent the occurrence of diseases and for their treatment is often referred to as apitherapy and is based mainly on the experience of the traditional system of medical practice in diverse ethnic communities. Today, a large number of studies are focused on the antitumor effects of BV, which are mainly attributed to its basic polypeptide melittin (MEL). Previous studies have indicated that BV and its major constituent MEL cause a strong toxic effect on different cancer cells, such as liver, lung, bladder, kidney, prostate, breast, and leukemia cells, while a less pronounced effect was observed in normal non-target cells. Their proposed mechanisms of action, such as the effect on proliferation and growth inhibition, cell cycle alterations, and induction of cell death through several cancer cell death mechanisms, are associated with the activation of phospholipase A2 (PLA2), caspases, and matrix metalloproteinases that destroy cancer cells. Numerous cellular effects of BV and MEL need to be elucidated on the molecular level, while the key issue has to do with the trigger of the apoptotic cascade. Apoptosis could be either a consequence of the plasmatic membrane fenestration or the result of the direct interaction of the BV components with pro-apoptotic and anti-apoptotic factors. The interaction of BV peptides and enzymes with the plasma membrane is a crucial step in the whole process. However, before its possible application as a remedy, it is crucial to identify the correct route of exposure and dosage of BV and MEL for potential therapeutic use as well as potential side effects on normal cells and tissues to avoid any possible adverse event.
Keywords: anticancer properties; apitherapy; apitoxin; bee venom; melittin; natural products; phospholipase A2; therapeutic application.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Harvey A. From Demons to Darlings: Drugs from Venoms. Drug Discov. Today. 1998;3:531–532. doi: 10.1016/S1359-6446(98)01269-0. - DOI
-
- von Reumont B.M., Anderluh G., Antunes A., Ayvazyan N., Beis D., Caliskan F., Crnković A., Damm M., Dutertre S., Ellgaard L., et al. Modern Venomics-Current Insights, Novel Methods, and Future Perspectives in Biological and Applied Animal Venom Research. Gigascience. 2022;11:giac048. doi: 10.1093/gigascience/giac048. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

