Exposure to Plasticiser DEHP Affects Eggs Spawned by Blue Mussels: A Possible Risk to Fertilisation?
- PMID: 38535905
- PMCID: PMC10974364
- DOI: 10.3390/toxics12030172
Exposure to Plasticiser DEHP Affects Eggs Spawned by Blue Mussels: A Possible Risk to Fertilisation?
Abstract
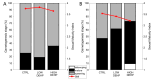
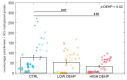
The endocrine disruptive chemical DEHP is a plasticiser often found in marine waters. Here, we assessed the effect of this additive on the number and size of eggs spawned by female mussels during a synchronised spawning event. After achieving the ripeness of the gonads, mussels of both sexes were exposed to two environmentally relevant concentrations of DEHP (nominal concentrations 0.5 and 50 µg/L) for one week. A spawning event was then induced and eggs were collected, counted, and their size measured (area and diameter). A slight but not significant effect was observed in lowering the number of eggs spawned when increasing the DEHP concentration. This effect was greater when adding spent gonads (possibly fully spawned females) to the total number of females. A significant effect of the lower dose on the average egg sizes was noticed, with a smaller area and diameter measured with respect to the control and the higher concentrated treatments. These results once again underline the importance for ecotoxicological studies to address the nonlinear dose-response effects of endocrine disruptive chemicals environmentally present at concentrations in the order of just a few µg/L that could not elicit a strong defence mechanism at low levels and be absorbed by filter feeder animals such as mussels.
Keywords: DEHP; blue mussels; egg count; egg size; fertility; reprotoxicity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Consequences of combined exposure to thermal stress and the plasticiser DEHP in Mytilus spp. differ by sex.Mar Pollut Bull. 2021 Sep;170:112624. doi: 10.1016/j.marpolbul.2021.112624. Epub 2021 Jun 16. Mar Pollut Bull. 2021. PMID: 34146859
-
Sex and gametogenesis stage are strong drivers of gene expression in Mytilus edulis exposed to environmentally relevant plasticiser levels and pH 7.7.Environ Sci Pollut Res Int. 2023 Feb;30(9):23437-23449. doi: 10.1007/s11356-022-23801-3. Epub 2022 Nov 2. Environ Sci Pollut Res Int. 2023. PMID: 36322353 Free PMC article.
-
Exposure to DEHP and MEHP from hatching to adulthood causes reproductive dysfunction and endocrine disruption in marine medaka (Oryzias melastigma).Aquat Toxicol. 2014 Jan;146:115-26. doi: 10.1016/j.aquatox.2013.10.025. Epub 2013 Nov 8. Aquat Toxicol. 2014. PMID: 24292025
-
Shellfish and residual chemical contaminants: hazards, monitoring, and health risk assessment along French coasts.Rev Environ Contam Toxicol. 2011;213:55-111. doi: 10.1007/978-1-4419-9860-6_3. Rev Environ Contam Toxicol. 2011. PMID: 21541848 Review.
-
The Minderoo-Monaco Commission on Plastics and Human Health.Ann Glob Health. 2023 Mar 21;89(1):23. doi: 10.5334/aogh.4056. eCollection 2023. Ann Glob Health. 2023. PMID: 36969097 Free PMC article. Review.
Cited by
-
The Threat of Bis(2-Ethylhexyl) Phthalate in Coastal and Marine Environments: Ecotoxicological Assays Using Tropical Species from Different Trophic Levels.Int J Environ Res Public Health. 2025 Mar 10;22(3):402. doi: 10.3390/ijerph22030402. Int J Environ Res Public Health. 2025. PMID: 40238434 Free PMC article.
References
-
- Soto A.M., Calabro J.M., Prechtl N.V., Yau A.Y., Orlando E.F., Daxenberger A., Kolok A.S., Guillette L.J., le Bizec B., Lange I.G., et al. Androgenic and Estrogenic Activity in Water Bodies Receiving Cattle Feedlot Effluent in Eastern Nebraska, USA. Environ. Health Perspect. 2004;112:346–352. doi: 10.1289/ehp.6590. - DOI - PMC - PubMed
-
- Aarab N., Lemaire-Gony S., Unruh E., Hansen P.D., Larsen B.K., Andersen O.K., Narbonne J.F. Preliminary Study of Responses in Mussel (Mytilus edilus) Exposed to Bisphenol A, Diallyl Phthalate and Tetrabromodiphenyl Ether. Aquat. Toxicol. 2006;78:86–92. doi: 10.1016/j.aquatox.2006.02.021. - DOI - PubMed
LinkOut - more resources
Full Text Sources

