Advancing Endocrine Disruptors via In Vitro Evaluation: Recognizing the Significance of the Organization for Economic Co-Operation and Development and United States Environmental Protection Agency Guidelines, Embracing New Assessment Methods, and the Urgent Need for a Comprehensive Battery of Tests
- PMID: 38535916
- PMCID: PMC10974109
- DOI: 10.3390/toxics12030183
Advancing Endocrine Disruptors via In Vitro Evaluation: Recognizing the Significance of the Organization for Economic Co-Operation and Development and United States Environmental Protection Agency Guidelines, Embracing New Assessment Methods, and the Urgent Need for a Comprehensive Battery of Tests
Abstract
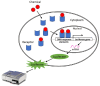
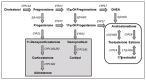
Efforts are being made globally to improve the evaluation and understanding of endocrine-disrupting chemicals. Recognition of their impact on human health and the environment has stimulated attention and research in this field. Various stakeholders, including scientists, regulatory agencies, policymakers, and industry representatives, are collaborating to develop robust methodologies and guidelines for assessing these disruptors. A key aspect of these efforts is the development of standardized testing protocols and guidelines that aim to provide consistent and reliable methods for identifying and characterizing endocrine disruptors. When evaluating the potential endocrine-disrupting activity of chemicals, no single test is capable of detecting all relevant endocrine-disrupting agents. The test battery approach is designed to reduce the risk of false negative results for compounds with toxic potential. A weight-of-evidence approach is therefore necessary for endocrine disruptor evaluation. This approach considers various types of data from multiple sources, assessing the overall strength, consistency, and reliability of the evidence. OECD guidelines are highly regarded for their scientific rigor, transparency, and consensus-based development process. It is crucial to explore and develop new methodologies that can effectively evaluate the risks associated with potential endocrine disruptors. Integrating these methods into a comprehensive weight-of-evidence framework will enhance risk assessments and facilitate informed decisions regarding the regulation and management of these substances, ensuring the protection of human health and the environment from their adverse effects.
Keywords: alternative method; endocrine disruptor; in vitro; validation; weight of evidence.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- WHO/IPCS (WHO, International Programme on Chemical Safety) Global Assessment of the State-of-the-Science of Endocrine Disruptors. WHO/PCS/EDC/02.2. 2002. [(accessed on 22 February 2024)]. Available online: https://www.who.int/publications/i/item/WHO-PSC-EDC-02.2.
Publication types
LinkOut - more resources
Full Text Sources

