Native Mass Spectrometry Dissects the Structural Dynamics of an Allosteric Heterodimer of SARS-CoV-2 Nonstructural Proteins
- PMID: 38535992
- PMCID: PMC11066969
- DOI: 10.1021/jasms.3c00453
Native Mass Spectrometry Dissects the Structural Dynamics of an Allosteric Heterodimer of SARS-CoV-2 Nonstructural Proteins
Abstract
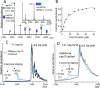
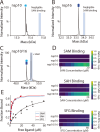
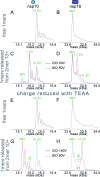
Structure-based drug design, which relies on precise understanding of the target protein and its interaction with the drug candidate, is dramatically expedited by advances in computational methods for candidate prediction. Yet, the accuracy needs to be improved with more structural data from high throughput experiments, which are challenging to generate, especially for dynamic and weak associations. Herein, we applied native mass spectrometry (native MS) to rapidly characterize ligand binding of an allosteric heterodimeric complex of SARS-CoV-2 nonstructural proteins (nsp) nsp10 and nsp16 (nsp10/16), a complex essential for virus survival in the host and thus a desirable drug target. Native MS showed that the dimer is in equilibrium with monomeric states in solution. Consistent with the literature, well characterized small cosubstrate, RNA substrate, and product bind with high specificity and affinity to the dimer but not the free monomers. Unsuccessfully designed ligands bind indiscriminately to all forms. Using neutral gas collision, the nsp16 monomer with bound cosubstrate can be released from the holo dimer complex, confirming the binding to nsp16 as revealed by the crystal structure. However, we observed an unusual migration of the endogenous zinc ions bound to nsp10 to nsp16 after collisional dissociation. The metal migration can be suppressed by using surface collision with reduced precursor charge states, which presumably resulted in minimal gas-phase structural rearrangement and highlighted the importance of complementary techniques. With minimal sample input (∼μg), native MS can rapidly detect ligand binding affinities and locations in dynamic multisubunit protein complexes, demonstrating the potential of an "all-in-one" native MS assay for rapid structural profiling of protein-to-AI-based compound systems to expedite drug discovery.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Varikoti R. A.; Schultz K. J.; Kombala C. J.; Kruel A.; Brandvold K. R.; Zhou M.; Kumar N. Integrated data-driven and experimental approaches to accelerate lead optimization targeting SARS-CoV-2 main protease. Journal of Computer-Aided Molecular Design 2023, 37 (8), 339–355. 10.1007/s10822-023-00509-1. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

