Effects of follicle-stimulating hormone on energy balance and tissue metabolic health after loss of ovarian function
- PMID: 38536037
- PMCID: PMC11208003
- DOI: 10.1152/ajpendo.00400.2023
Effects of follicle-stimulating hormone on energy balance and tissue metabolic health after loss of ovarian function
Abstract
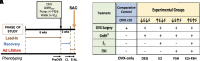
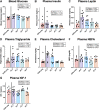
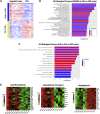
Loss of ovarian function imparts increased susceptibility to obesity and metabolic disease. These effects are largely attributed to decreased estradiol (E2), but the role of increased follicle-stimulating hormone (FSH) in modulating energy balance has not been fully investigated. Previous work that blocked FSH binding to its receptor in mice suggested this hormone may play a part in modulating body weight and energy expenditure after ovariectomy (OVX). We used an alternate approach to isolate the individual and combined contributions of FSH and E2 in mediating energy imbalance and changes in tissue-level metabolic health. Female Wistar rats were ovariectomized and given the gonadotropin releasing hormone (GnRH) antagonist degarelix to suppress FSH production. E2 and FSH were then added back individually and in combination for a period of 3 wk. Energy balance, body mass composition, and transcriptomic profiles of individual tissues were obtained. In contrast to previous studies, suppression and replacement of FSH in our paradigm had no effect on body weight, body composition, food intake, or energy expenditure. We did, however, observe organ-specific effects of FSH that produced unique transcriptomic signatures of FSH in retroperitoneal white adipose tissue. These included reductions in biological processes related to lipogenesis and carbohydrate transport. In addition, rats administered FSH had reduced liver triglyceride concentration (P < 0.001), which correlated with FSH-induced changes at the transcriptomic level. Although not appearing to modulate energy balance after loss of ovarian function in rats, FSH may still impart tissue-specific effects in the liver and white adipose tissue that might affect the metabolic health of those organs.NEW & NOTEWORTHY We find no effect of follicle-stimulating hormone (FSH) on energy balance using a novel model in which rats are ovariectomized, subjected to gonadotropin-releasing hormone antagonism, and systematically given back FSH by osmotic pump. However, tissue-specific effects of FSH on adipose tissue and liver were observed in this study. These include unique transcriptomic signatures induced by the hormone and a stark reduction in hepatic triglyceride accumulation.
Keywords: adipose tissue; energy balance; follicle-stimulating hormone; lipids; liver.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
- I01 BX005135/BX/BLRD VA/United States
- IK2 BX004533/BX/BLRD VA/United States
- Donation/Makowski Family Endowment
- U54 AG062319/AG/NIA NIH HHS/United States
- F32 DK126312/DK/NIDDK NIH HHS/United States
- T32DK120521/HHS | National Institutes of Health (NIH)
- U54AG062319/HHS | National Institutes of Health (NIH)
- P30DK048520/HHS | National Institutes of Health (NIH)
- R01 HD103384/HD/NICHD NIH HHS/United States
- F32DK126312/HHS | National Institutes of Health (NIH)
- T32 DK120521/DK/NIDDK NIH HHS/United States
- P30 DK048520/DK/NIDDK NIH HHS/United States
- 110/CU | Center for Women's Health Research, University of Colorado (CWHR, UC)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

