A Phase I Trial of Enzalutamide Plus Selective Glucocorticoid Receptor Modulator Relacorilant in Patients with Metastatic Castration-Resistant Prostate Cancer
- PMID: 38536082
- PMCID: PMC11147727
- DOI: 10.1158/1078-0432.CCR-23-3636
A Phase I Trial of Enzalutamide Plus Selective Glucocorticoid Receptor Modulator Relacorilant in Patients with Metastatic Castration-Resistant Prostate Cancer
Abstract
Purpose: The majority of patients with metastatic prostate cancer who receive androgen-deprivation therapy and androgen receptor (AR) signaling inhibitors (ARSI) progress. Activation of the glucocorticoid receptor (GR) is associated with ARSI resistance. This single-arm phase I trial assessed safety and pharmacokinetic (PK) feasibility of a combined AR antagonist (enzalutamide) and selective GR modulator (relacorilant) in patients with metastatic castration-resistant prostate cancer (mCRPC).
Patients and methods: This was a phase I trial (NCT03674814) of relacorilant and enzalutamide in patients with refractory mCRPC enrolled using a 6+3 design. The enzalutamide dose was kept constant at 120 mg/d with escalating doses of relacorilant based on safety and PK measures in cohorts of ≥6 patients. The primary objective was safety and establishment of pharmacologically active doses. Secondary objectives were related to antitumor activity.
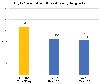
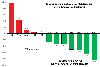
Results: Thirty-five patients with mCRPC were enrolled. Twenty-three were accrued across three dose cohorts in the dose-escalation phase, and 12 enrolled at the recommended phase II dose. The combination was generally well tolerated, safe, and achieved desirable enzalutamide PK. RP2D of 120 + 150 mg/d, respectively, was established. Median time on study was 2.2 months with four patients remaining on study for longer than 11 months. Four of 12 evaluable patients had a prostate-specific antigen (PSA) partial response.
Conclusions: This is the first prospective trial combining an AR antagonist and a nonsteroidal selective GR modulator. The combination was safe and well tolerated with PSA response and prolonged disease control observed in a limited subset of patients. Further prospective trials are justified to evaluate efficacy and identify predictive biomarkers of response.
©2024 American Association for Cancer Research.
Figures
Similar articles
-
Enzalutamide for the treatment of nonmetastatic castration-resistant prostate cancer.Expert Opin Pharmacother. 2020 Dec;21(17):2091-2099. doi: 10.1080/14656566.2020.1803281. Epub 2020 Aug 12. Expert Opin Pharmacother. 2020. PMID: 32783772 Review.
-
A Phase Ib/IIa Study of the Pan-BET Inhibitor ZEN-3694 in Combination with Enzalutamide in Patients with Metastatic Castration-resistant Prostate Cancer.Clin Cancer Res. 2020 Oct 15;26(20):5338-5347. doi: 10.1158/1078-0432.CCR-20-1707. Epub 2020 Jul 21. Clin Cancer Res. 2020. PMID: 32694156 Free PMC article. Clinical Trial.
-
Phase I/II Trial of Enzalutamide and Mifepristone, a Glucocorticoid Receptor Antagonist, for Metastatic Castration-Resistant Prostate Cancer.Clin Cancer Res. 2022 Apr 14;28(8):1549-1559. doi: 10.1158/1078-0432.CCR-21-4049. Clin Cancer Res. 2022. PMID: 35110415 Free PMC article. Clinical Trial.
-
Enzalutamide: a review of its use in metastatic, castration-resistant prostate cancer.Drugs. 2013 Oct;73(15):1723-32. doi: 10.1007/s40265-013-0129-9. Drugs. 2013. PMID: 24127223 Review.
-
Molecular characterization of enzalutamide-treated bone metastatic castration-resistant prostate cancer.Eur Urol. 2015 Jan;67(1):53-60. doi: 10.1016/j.eururo.2014.05.005. Epub 2014 May 29. Eur Urol. 2015. PMID: 24882673 Free PMC article. Clinical Trial.
Cited by
-
New advances of the androgen receptor in prostate cancer: report from the 1st International Androgen Receptor Symposium.J Transl Med. 2024 Jan 18;22(1):71. doi: 10.1186/s12967-024-04878-5. J Transl Med. 2024. PMID: 38238739 Free PMC article.
-
Androgen receptor-mediated assisted loading of the glucocorticoid receptor modulates transcriptional responses in prostate cancer cells.Genome Res. 2025 Aug 1;35(8):1717-1732. doi: 10.1101/gr.280224.124. Genome Res. 2025. PMID: 40456604 Free PMC article.
-
Glucocorticoid receptor action in prostate cancer: the role of transcription factor crosstalk.Front Endocrinol (Lausanne). 2024 Jul 4;15:1437179. doi: 10.3389/fendo.2024.1437179. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39027480 Free PMC article. Review.
-
Steroid hormones as modulators of anti-tumoural immunity.Nat Rev Endocrinol. 2025 Jun;21(6):331-343. doi: 10.1038/s41574-025-01102-2. Epub 2025 Mar 24. Nat Rev Endocrinol. 2025. PMID: 40128599 Review.
References
-
- Davis ID, et al., Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N Engl J Med, 2019. 381(2): p. 121–131. - PubMed
-
- Desai SJ, et al., Inappropriate activation of the androgen receptor by nonsteroids: involvement of the Src kinase pathway and its therapeutic implications. Cancer Res, 2006. 66(21): p. 10449–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous