Validation of the VisionArray® Chip Assay for HPV DNA Testing in Histology Specimens of Oropharyngeal Squamous Cell Carcinoma
- PMID: 38536624
- PMCID: PMC10973319
- DOI: 10.1007/s12105-024-01628-3
Validation of the VisionArray® Chip Assay for HPV DNA Testing in Histology Specimens of Oropharyngeal Squamous Cell Carcinoma
Abstract
Background: The detection of human papillomavirus (HPV) has several implications in the diagnostic work-up and management of oropharyngeal squamous cell carcinoma (OPSCC). The choice of HPV detection assay and testing algorithms differ across institutions and vary in cost, detection targets, technical feasibility, and turnaround time. In this study, we aimed to validate the VisionArray® HPV Chip for formalin-fixed and paraffin-embedded (FFPE) samples of OPSCC using the previously applied standard pan-HPV DNA PCR assay as a reference.
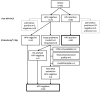
Methods: The validation cohort consisted of FFPE tissue samples from patients previously diagnosed with HPV DNA-positive OPSCC (n = 80), HPV DNA-negative OPSCC (n = 21), and a benign group of tumor samples consisting of Warthin's tumors (n = 20) and branchial cleft cysts of the lateral neck (n = 14). All samples were tested with p16 immunohistochemistry, pan-HPV DNA PCR, and the VisionArray® HPV Chip.
Results: The overall sensitivity and specificity of the VisionArray® HPV Chip assay were 100% [95% CI 95.5%; 100.0%] and 96.3% [95% CI 87.3%; 99.6%] and the positive predictive value and negative predictive value were 97.6% [95% CI 91.5%; 99.7%] and 100% [95% CI 93.2%; 100%], respectively.
Conclusions: The VisionArray® HPV Chip assay can be recommended for high-risk HPV testing in FFPE tissue samples from OPSCC, providing both a fast and simultaneous genotyping for 41 clinically relevant HPV types.
Keywords: HPV DNA; HPV genotyping; Human papillomavirus; Oropharyngeal cancer; Squamous cell carcinoma; VisionArray.
© 2024. The Author(s).
Conflict of interest statement
All authors declare no conflicts of interest. VisionArray® is a registered trademark by ZytoVision. The company had no role in the funding or in the conduction of the study.
Figures


References
-
- Channir HI, Lomholt AF, Gerds TA et al (2022) Human papillomavirus testing in metastatic squamous cell carcinoma of the neck with unknown primary using PCR on fine-needle aspiration smears: a prospective clinical study. Eur Arch Oto Rhino Laryngol 279:3115–3121 - PubMed
-
- Mehanna H, Taberna M, von Buchwald C et al (2023) Prognostic implications of p16 and HPV discordance in oropharyngeal cancer (HNCIG-EPIC-OPC): a multicentre, multinational, individual patient data analysis. Lancet Oncol 24:239–251 - PubMed
-
- Lewis JS, Beadle B, Bishop JA et al (2018) Human papillomavirus testing in head and neck carcinomas guideline from the college of american pathologists. Arch Pathol Lab Med 142:559–597 - PubMed
-
- Channir HI, GrønhøjLarsen C, Ahlborn LB et al (2016) Validation study of HPV DNA detection from stained FNA smears by polymerase chain reaction: Improving the diagnostic workup of patients with a tumor on the neck. Cancer Cytopathol 124:820–827 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

