Refractive Error Change and Overminus Lens Therapy for Childhood Intermittent Exotropia
- PMID: 38536764
- PMCID: PMC10958385
- DOI: 10.1001/jamaophthalmol.2024.0276
Refractive Error Change and Overminus Lens Therapy for Childhood Intermittent Exotropia
Abstract
Importance: Increased myopic shift was found to be associated with 1 year of overminus spectacle treatment for children with intermittent exotropia (IXT). Persistence of myopic shift after discontinuing overminus spectacles is unknown.
Objective: To compare refractive error change over 3 years in children with IXT originally treated with overminus vs nonoverminus spectacles.
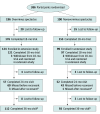
Design, setting, and participants: This study was an 18-month extension of the Trial of Overminus Spectacle Therapy for Intermittent Exotropia cohort, which previously randomized children aged 3 to 10 years with IXT and baseline spherical equivalent refractive error (SER) between -6.00 diopters (D) and 1.00 D to overminus spectacles (-2.50 D for 12 months, -1.25 D for 3 months, and nonoverminus for 3 months) or nonoverminus spectacles. Children were recruited from 56 sites from July 2010 to February 2022. Data were analyzed from February 2022 to January 2024.
Interventions: After trial completion at 18 months, participants were followed up at 24 and 36 months. Treatment was at investigator discretion from 18 to 36 months.
Main outcomes and measures: Change in SER (cycloplegic retinoscopy) from baseline to 36 months.
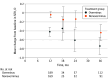
Results: Of 386 children in the Trial of Overminus Spectacle Therapy for Intermittent Exotropia, 223 (57.8%) consented to 18 months of additional follow-up, including 124 of 196 (63.3%) in the overminus treatment group and 99 of 190 (52.1%) in the nonoverminus treatment group. Of 205 children who completed 36-month follow-up, 116 (56.6%) were female, and the mean (SD) age at randomization was 6.2 (2.1) years. Mean (SD) SER change from baseline to 36 months was greater in the overminus group (-0.74 [1.00] D) compared with the nonoverminus group (-0.44 [0.85] D; adjusted difference, -0.36 D; 95% CI, -0.59 to -0.12; P = .003), with 30 of 112 (26.8%) in the overminus group having more than 1 D of myopic shift compared with 14 of 91 (15%) in the nonoverminus group (risk ratio, 1.8; 95% CI, 1.0-3.0). From 12 to 36 months, mean (SD) myopic shift was -0.34 (0.67) D and -0.36 (0.66) D in the overminus and nonoverminus groups, respectively (adjusted difference, -0.001 D; 95% CI, -0.18 to 0.18; P = .99).
Conclusions and relevance: The greater myopic shift observed after 1 year of -2.50-D overminus lens treatment remained at 3 years. Both groups had similar myopic shift during the 2-year period after treatment weaning and cessation. The risk of myopic shift should be discussed with parents when considering overminus lens treatment.
Trial registration: ClinicalTrials.gov Identifier: NCT02807350.
Conflict of interest statement
Figures
References
-
- McKean-Cowdin R, Cotter SA, Tarczy-Hornoch K, et al. ; Multi-Ethnic Pediatric Eye Disease Study Group . Prevalence of amblyopia or strabismus in Asian and non-Hispanic White preschool children: Multi-Ethnic Pediatric Eye Disease study. Ophthalmology. 2013;120(10):2117-2124. doi: 10.1016/j.ophtha.2013.03.001 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

