Dynamics of leaching of POPs and additives from plastic in a Procellariiform gastric model: Diet- and polymer-dependent effects and implications for long-term exposure
- PMID: 38536858
- PMCID: PMC10971572
- DOI: 10.1371/journal.pone.0299860
Dynamics of leaching of POPs and additives from plastic in a Procellariiform gastric model: Diet- and polymer-dependent effects and implications for long-term exposure
Abstract
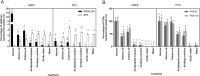
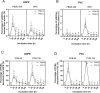
Procellariiform seabirds are known to have high rates of plastic ingestion. We investigated the bioaccessibility of plastic-associated chemicals [plastic additives and sorbed persistent organic pollutants (POPs)] leached from plastic over time using an in vitro Procellariiform gastric model. High-density polyethylene (HDPE) and polyvinyl chloride (PVC), commonly ingested by Procellariiform seabirds, were manufactured with one additive [decabrominated diphenyl ether (PBDE-209) or bisphenol S (BPS)]. HDPE and PVC added with PBDE-209 were additionally incubated in salt water with 2,4,4'-trichloro-1,1'-biphenyl (PCB-28) and 2,2',3,4,4',5'-hexachlorobiphenyl (PCB-138) to simulate sorption of POPs on plastic in the marine environment. Our results indicate that the type of plastic (nature of polymer and additive), presence of food (i.e., lipids and proteins) and gastric secretions (i.e., pepsin) influence the leaching of chemicals in a seabird. In addition, 100% of the sorbed POPs were leached from the plastic within 100 hours, while only 2-5% of the additives were leached from the matrix within 100 hours, suggesting that the remaining 95% of the additives could continue to be leached. Overall, our study illustrates how plastic type, diet and plastic retention time can influence a Procellariform's exposure risk to plastic-associated chemicals.
Copyright: © 2024 Van Hassel et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Ryan PG. Ingestion of Plastics by Marine Organisms. Handb Environ Chem. 2019;78:235–66.
-
- Furness RW. Ingestion of plastic particles by seabirds at Gough Island, South Atlantic Ocean. Environ Pollution Ser A, Ecol Biol. 1985;38(3):261–72.
-
- Pierce KE, Harris RJ, Larned LS, Pokras MA. Obstruction and starvation associated with plastic ingestion in a Northern Gannet Morus bassanus and a Greater Shearwater Puffinus gravis. Mar Ornithol. 2004;32(2):187–9.

