Conformation selection by ATP-competitive inhibitors and allosteric communication in ERK2
- PMID: 38537148
- PMCID: PMC10972564
- DOI: 10.7554/eLife.91507
Conformation selection by ATP-competitive inhibitors and allosteric communication in ERK2
Abstract
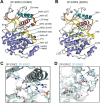
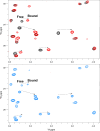
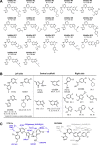
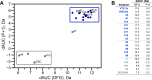
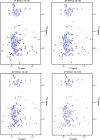
Activation of the extracellular signal-regulated kinase-2 (ERK2) by phosphorylation has been shown to involve changes in protein dynamics, as determined by hydrogen-deuterium exchange mass spectrometry (HDX-MS) and NMR relaxation dispersion measurements. These can be described by a global exchange between two conformational states of the active kinase, named 'L' and 'R,' where R is associated with a catalytically productive ATP-binding mode. An ATP-competitive ERK1/2 inhibitor, Vertex-11e, has properties of conformation selection for the R-state, revealing movements of the activation loop that are allosterically coupled to the kinase active site. However, the features of inhibitors important for R-state selection are unknown. Here, we survey a panel of ATP-competitive ERK inhibitors using HDX-MS and NMR and identify 14 new molecules with properties of R-state selection. They reveal effects propagated to distal regions in the P+1 and helix αF segments surrounding the activation loop, as well as helix αL16. Crystal structures of inhibitor complexes with ERK2 reveal systematic shifts in the Gly loop and helix αC, mediated by a Tyr-Tyr ring stacking interaction and the conserved Lys-Glu salt bridge. The findings suggest a model for the R-state involving small movements in the N-lobe that promote compactness within the kinase active site and alter mobility surrounding the activation loop. Such properties of conformation selection might be exploited to modulate the protein docking interface used by ERK substrates and effectors.
Keywords: E. coli; MAP kinase; NMR; biochemistry; cancer therapeutics; chemical biology; conformation selection; hydrogen exchange; inhibitor.
© 2023, Anderson et al.
Conflict of interest statement
JA, DV, DJ, LP, NA No competing interests declared, GV Guy Vigers is Structural Biology Consultant at Allium Consulting LLC, HC Dr. Huifen Chen is Distinguished Scientist at Genentech, Inc, JM Dr. John Moffat is Distinguished Scientist at Genentech, Inc
Figures














Update of
-
Conformation Selection by ATP-competitive Inhibitors and Allosteric Communication in ERK2.bioRxiv [Preprint]. 2023 Nov 6:2023.09.12.557258. doi: 10.1101/2023.09.12.557258. bioRxiv. 2023. Update in: Elife. 2024 Mar 27;12:RP91507. doi: 10.7554/eLife.91507. PMID: 37745518 Free PMC article. Updated. Preprint.
References
-
- Blake JF, Burkard M, Chan J, Chen H, Chou KJ, Diaz D, Dudley DA, Gaudino JJ, Gould SE, Grina J, Hunsaker T, Liu L, Martinson M, Moreno D, Mueller L, Orr C, Pacheco P, Qin A, Rasor K, Ren L, Robarge K, Shahidi-Latham S, Stults J, Sullivan F, Wang W, Yin J, Zhou A, Belvin M, Merchant M, Moffat J, Schwarz JB. Discovery of (S)-1-(1-(4-chloro-3-fluorophenyl)-2-hydroxyethyl)-4-(2-((1-methyl-1H-pyrazol-5-yl)amino)pyrimidin-4-yl)pyridin-2(1H)-one (GDC-0994), an extracellular signal-regulated kinase 1/2 (ERK1/2) inhibitor in early clinical development. Journal of Medicinal Chemistry. 2016;59:5650–5660. doi: 10.1021/acs.jmedchem.6b00389. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- R01 GM114594/GM/NIGMS NIH HHS/United States
- S10OD025267/NH/NIH HHS/United States
- S10 RR026641/RR/NCRR NIH HHS/United States
- R01GM114594/NH/NIH HHS/United States
- S10RR026641/NH/NIH HHS/United States
- S10 OD025267/OD/NIH HHS/United States
- T32 GM142607/GM/NIGMS NIH HHS/United States
- S10 OD025020/OD/NIH HHS/United States
- R35 GM136392/GM/NIGMS NIH HHS/United States
- P30CA046934/NH/NIH HHS/United States
- P30 CA046934/CA/NCI NIH HHS/United States
- S10OD025020/NH/NIH HHS/United States
- S10 OD034218/OD/NIH HHS/United States
- R35GM136392/NH/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

