Ophthalmate is a new regulator of motor functions via CaSR: implications for movement disorders
- PMID: 38537648
- PMCID: PMC11449132
- DOI: 10.1093/brain/awae097
Ophthalmate is a new regulator of motor functions via CaSR: implications for movement disorders
Abstract
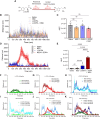
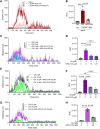
Dopamine's role as the principal neurotransmitter in motor functions has long been accepted. We broaden this conventional perspective by demonstrating the involvement of non-dopaminergic mechanisms. In mouse models of Parkinson's disease, we observed that L-DOPA elicited a substantial motor response even when its conversion to dopamine was blocked by inhibiting the enzyme aromatic amino acid decarboxylase (AADC). Remarkably, the motor activity response to L-DOPA in the presence of an AADC inhibitor (NSD1015) showed a delayed onset, yet greater intensity and longer duration, peaking at 7 h, compared to when L-DOPA was administered alone. This suggests an alternative pathway or mechanism, independent of dopamine signalling, mediating the motor functions. We sought to determine the metabolites associated with the pronounced hyperactivity observed, using comprehensive metabolomics analysis. Our results revealed that the peak in motor activity induced by NSD1015/L-DOPA in Parkinson's disease mice is associated with a surge (20-fold) in brain levels of the tripeptide ophthalmic acid (also known as ophthalmate in its anionic form). Interestingly, we found that administering ophthalmate directly to the brain rescued motor deficits in Parkinson's disease mice in a dose-dependent manner. We investigated the molecular mechanisms underlying ophthalmate's action and discovered, through radioligand binding and cAMP-luminescence assays, that ophthalmate binds to and activates the calcium-sensing receptor (CaSR). Additionally, our findings demonstrated that a CaSR antagonist inhibits the motor-enhancing effects of ophthalmate, further solidifying the evidence that ophthalmate modulates motor functions through the activation of the CaSR. The discovery of ophthalmate as a novel regulator of motor function presents significant potential to transform our understanding of brain mechanisms of movement control and the therapeutic management of related disorders.
Keywords: CaSR; Parkinson’s disease; dopamine; motor; ophthalmate.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
A.A. and O.C. are inventors on a pending patent application that covers the role of ophthalmate and CaSR in motor functions. The remaining authors declare no competing interests.
Figures




Similar articles
-
Locomotor response to L-DOPA in reserpine-treated rats following central inhibition of aromatic L-amino acid decarboxylase: further evidence for non-dopaminergic actions of L-DOPA and its metabolites.Neurosci Res. 2010 Sep;68(1):44-50. doi: 10.1016/j.neures.2010.06.003. Epub 2010 Jun 11. Neurosci Res. 2010. PMID: 20542064
-
Dual effects of L-3,4-dihydroxyphenylalanine on aromatic L-amino acid decarboxylase, dopamine release and motor stimulation in the reserpine-treated rat: evidence that behaviour is dopamine independent.Neuroscience. 2000;95(1):97-111. doi: 10.1016/s0306-4522(99)00406-6. Neuroscience. 2000. PMID: 10619466
-
Aromatic L-amino acid decarboxylase immunoreactive cells in the rat striatum: a possible site for the conversion of exogenous L-DOPA to dopamine.Brain Res. 1995 Dec 15;704(1):51-60. doi: 10.1016/0006-8993(95)01104-8. Brain Res. 1995. PMID: 8750961
-
Expanding the repertoire of L-DOPA's actions: A comprehensive review of its functional neurochemistry.Prog Neurobiol. 2017 Apr;151:57-100. doi: 10.1016/j.pneurobio.2016.07.002. Epub 2016 Jul 4. Prog Neurobiol. 2017. PMID: 27389773 Review.
-
Potential Therapeutic Approach using Aromatic l-amino Acid Decarboxylase and Glial-derived Neurotrophic Factor Therapy Targeting Putamen in Parkinson's Disease.Curr Gene Ther. 2024;24(4):278-291. doi: 10.2174/0115665232283842240102073002. Curr Gene Ther. 2024. PMID: 38310455 Review.
Cited by
-
Levodopa treatment: impacts and mechanisms throughout Parkinson's disease progression.J Neural Transm (Vienna). 2025 Jun;132(6):743-779. doi: 10.1007/s00702-025-02893-4. Epub 2025 Apr 11. J Neural Transm (Vienna). 2025. PMID: 40214767 Free PMC article. Review.
References
-
- Bartholini G, Burkard WP, Pletscher A, Bates HM. Increase of cerebral catecholamines caused by 3,4-dihydroxyphenylalanine after inhibition of peripheral decarboxylase. Nature. 1967;215:852–853. - PubMed
-
- Hornykiewicz O. The mechanisms of action of L-dopa in Parkinson’s disease. Life Sci. 1974;15:1249–1259. - PubMed
-
- Papavasiliou PS, Cotzias GC, Duby SE, Steck AJ, Fehling C, Bell MA. Levodopa in parkinsonism: Potentiation of central effects with a peripheral inhibitor. N Engl J Med. 1972;286:8–14. - PubMed
-
- Lieberman A, Goodgold A, Jonas S, Leibowitz M. Comparison of dopa decarboxylase inhibitor (carbidopa) combined with levodopa and levodopa alone in Parkinson’s disease. Neurology. 1975;25:911–916. - PubMed
-
- Grotzsch H, Sztajzel R, Burkhard PR. Levodopa-induced ocular dyskinesia in Parkinson’s disease. Eur J Neurol. 2007;14:1124–1128. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

