Central visual pathways affected by degenerative retinal disease before and after gene therapy
- PMID: 38538211
- PMCID: PMC11370797
- DOI: 10.1093/brain/awae096
Central visual pathways affected by degenerative retinal disease before and after gene therapy
Abstract
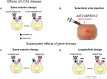
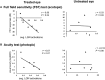
Genetic diseases affecting the retina can result in partial or complete loss of visual function. Leber's congenital amaurosis (LCA) is a rare blinding disease, usually inherited in an autosomally recessive manner, with no cure. Retinal gene therapy has been shown to improve vision in LCA patients caused by mutations in the RPE65 gene (LCA2). However, little is known about how activity in central visual pathways is affected by the disease or by subsequent gene therapy. Functional MRI (fMRI) was used to assess retinal signal transmission in cortical and subcortical visual structures before and 1 year after retinal intervention. The fMRI paradigm consisted of 15-s blocks of flickering (8 Hz) black and white checkerboards interleaved with 15 s of blank (black) screen. Visual activation in the brain was assessed using the general linear model, with multiple comparisons corrected using the false discovery rate method. Response to visual stimulation through untreated eyes of LCA2 patients showed heightened fMRI responses in the superior colliculus and diminished activities in the lateral geniculate nucleus (LGN) compared to controls, indicating a shift in the patients' visual processing towards the retinotectal pathway. Following gene therapy, stimuli presented to the treated eye elicited significantly stronger fMRI responses in the LGN and primary visual cortex, indicating some re-engagement of the geniculostriate pathway (GS) pathway. Across patients, the post-treatment LGN fMRI responses correlated significantly with performance on a clinical test measuring light sensitivity. Our results demonstrate that the low vision observed in LCA2 patients involves a shift in visual processing toward the retinotectal pathway, and that gene therapy partially reinstates visual transmission through the GS pathway. This selective boosting of retinal output through the GS pathway and its correlation to improved visual performance, following several years of degenerative retinal disease, is striking. However, while retinal gene therapy and other ocular interventions have given hope to RPE65 patients, it may take years before development of therapies tailored to treat the diseases in other low vision patients are available. Our demonstration of a shift toward the retinotectal pathway in these patients may spur the development of new tools and rehabilitation strategies to help maximize the use of residual visual abilities and augment experience-dependent plasticity.
Keywords: brain plasticity; geniculostriate pathway; retinal gene therapy; retinotectal pathway; visual system.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
J.B. reports that she and her husband were co-authors on a patent licensed by Spark Therapeutics that described retinal gene therapy in LCA2 patients but that they waived any potential financial gain in 2002. J.B. consulted for Spark Therapeutics on preclinical data and how it could relate to delivery of voretigene neparvovec-rzyl to patients in Canada and Japan. J.B. reports serving on the boards and scientific advisory boards of several different gene therapy companies. She also has some intellectual property that is managed by the University of Pennsylvania.
Figures



Similar articles
-
The Role of the Human Visual Cortex in Assessment of the Long-Term Durability of Retinal Gene Therapy in Follow-on RPE65 Clinical Trial Patients.Ophthalmology. 2017 Jun;124(6):873-883. doi: 10.1016/j.ophtha.2017.01.029. Epub 2017 Feb 23. Ophthalmology. 2017. PMID: 28237426 Free PMC article. Clinical Trial.
-
Canine and human visual cortex intact and responsive despite early retinal blindness from RPE65 mutation.PLoS Med. 2007 Jun;4(6):e230. doi: 10.1371/journal.pmed.0040230. PLoS Med. 2007. PMID: 17594175 Free PMC article.
-
Plasticity of the human visual system after retinal gene therapy in patients with Leber's congenital amaurosis.Sci Transl Med. 2015 Jul 15;7(296):296ra110. doi: 10.1126/scitranslmed.aaa8791. Sci Transl Med. 2015. PMID: 26180100 Free PMC article.
-
The effect of human gene therapy for RPE65-associated Leber's congenital amaurosis on visual function: a systematic review and meta-analysis.Orphanet J Rare Dis. 2020 Feb 14;15(1):49. doi: 10.1186/s13023-020-1304-1. Orphanet J Rare Dis. 2020. PMID: 32059734 Free PMC article.
-
Gene therapy for Leber congenital amaurosis: advances and future directions.Graefes Arch Clin Exp Ophthalmol. 2012 Aug;250(8):1117-28. doi: 10.1007/s00417-012-2028-2. Epub 2012 May 29. Graefes Arch Clin Exp Ophthalmol. 2012. PMID: 22644094 Free PMC article. Review.
Cited by
-
Characterizing Inner Retinal Changes in End-Stage Inherited Retinal Diseases That Might be Suitable for Optogenetic Therapies.Transl Vis Sci Technol. 2025 Jun 2;14(6):2. doi: 10.1167/tvst.14.6.2. Transl Vis Sci Technol. 2025. PMID: 40455038 Free PMC article.
References
-
- Perry VH, Oehler R, Cowey A. Retinal ganglion cells that project to the dorsal lateral geniculate nucleus in the macaque monkey. Neuroscience. 1984;12:1101–1123. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

