Blebology: principles of bleb-based migration
- PMID: 38538441
- PMCID: PMC11424778
- DOI: 10.1016/j.tcb.2024.02.009
Blebology: principles of bleb-based migration
Abstract
Bleb-based migration, a conserved cell motility mode, has a crucial role in both physiological and pathological processes. Unlike the well-elucidated mechanisms of lamellipodium-based mesenchymal migration, the dynamics of bleb-based migration remain less understood. In this review, we highlight in a systematic way the establishment of front-rear polarity, bleb formation and extension, and the distinct regimes of bleb dynamics. We emphasize new evidence proposing a regulatory role of plasma membrane-cortex interactions in blebbing behavior and discuss the generation of force and its transmission during migration. Our analysis aims to deepen the understanding of the physical and molecular mechanisms of bleb-based migration, shedding light on its implications and significance for health and disease.
Keywords: actomyosin; amoeboid migration; bleb migration; cell polarity; small GTPase.
Copyright © 2024 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence this review.
Figures
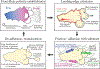



References
-
- Krause M and Gautreau A (2014) Steering cell migration: lamellipodium dynamics and the regulation of directional persistence. Nat Rev Mol Cell Biol 15, 577–590 - PubMed
-
- Bailly M and Condeelis J (2002) Cell motility: insights from the backstage. Nat Cell Biol 4, E292–E294 - PubMed
-
- Bodor DL et al. (2020) Of Cell Shapes and Motion: The Physical Basis of Animal Cell Migration. Developmental Cell 52, 550–562 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

