Expected and unexpected effects after systemic inhibition of Hippo transcriptional output in cancer
- PMID: 38538573
- PMCID: PMC10973481
- DOI: 10.1038/s41467-024-46531-1
Expected and unexpected effects after systemic inhibition of Hippo transcriptional output in cancer
Abstract
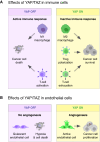
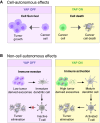
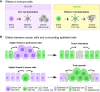
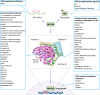
Hyperactivation of YAP/TAZ, the Hippo pathway downstream effectors, is common in human cancer. The requirement of YAP/TAZ for cancer cell survival in preclinical models, prompted the development of pharmacological inhibitors that suppress their transcriptional activity. However, systemic YAP/TAZ inhibition may sometimes have unpredictable patient outcomes, with limited or even adverse effects because YAP/TAZ action is not simply tumor promoting but also tumor suppressive in some cell types. Here, we review the role of the Hippo pathway in distinct tumor cell populations, discuss the impact of inhibiting Hippo output on tumor growth, and examine current developments in YAP/TAZ inhibitors.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Tang TT, Post L. Abstract 5364: The TEAD autopalmitoylation inhibitor VT3989 improves efficacy and increases durability of efficacy of osimertinib in preclinical EGFR mutant tumor models. Cancer Res. 2022;82:5364–5364. doi: 10.1158/1538-7445.AM2022-5364. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

