CGRP sensory neurons promote tissue healing via neutrophils and macrophages
- PMID: 38538784
- PMCID: PMC11023938
- DOI: 10.1038/s41586-024-07237-y
CGRP sensory neurons promote tissue healing via neutrophils and macrophages
Abstract
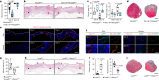
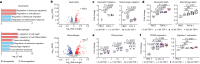
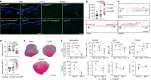
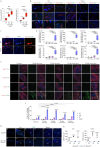
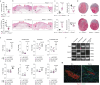
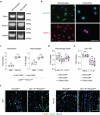
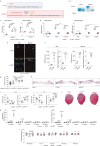
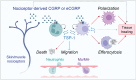
The immune system has a critical role in orchestrating tissue healing. As a result, regenerative strategies that control immune components have proved effective1,2. This is particularly relevant when immune dysregulation that results from conditions such as diabetes or advanced age impairs tissue healing following injury2,3. Nociceptive sensory neurons have a crucial role as immunoregulators and exert both protective and harmful effects depending on the context4-12. However, how neuro-immune interactions affect tissue repair and regeneration following acute injury is unclear. Here we show that ablation of the NaV1.8 nociceptor impairs skin wound repair and muscle regeneration after acute tissue injury. Nociceptor endings grow into injured skin and muscle tissues and signal to immune cells through the neuropeptide calcitonin gene-related peptide (CGRP) during the healing process. CGRP acts via receptor activity-modifying protein 1 (RAMP1) on neutrophils, monocytes and macrophages to inhibit recruitment, accelerate death, enhance efferocytosis and polarize macrophages towards a pro-repair phenotype. The effects of CGRP on neutrophils and macrophages are mediated via thrombospondin-1 release and its subsequent autocrine and/or paracrine effects. In mice without nociceptors and diabetic mice with peripheral neuropathies, delivery of an engineered version of CGRP accelerated wound healing and promoted muscle regeneration. Harnessing neuro-immune interactions has potential to treat non-healing tissues in which dysregulated neuro-immune interactions impair tissue healing.
© 2024. The Author(s).
Conflict of interest statement
Y.-Z.L. and M.M.M. are named as inventors on a patent application by Monash University relating to the molecular design described here. The other authors declare no competing interests.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

