A brainstem-hypothalamus neuronal circuit reduces feeding upon heat exposure
- PMID: 38538787
- PMCID: PMC11041654
- DOI: 10.1038/s41586-024-07232-3
A brainstem-hypothalamus neuronal circuit reduces feeding upon heat exposure
Abstract
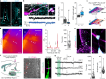
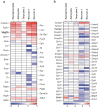
Empirical evidence suggests that heat exposure reduces food intake. However, the neurocircuit architecture and the signalling mechanisms that form an associative interface between sensory and metabolic modalities remain unknown, despite primary thermoceptive neurons in the pontine parabrachial nucleus becoming well characterized1. Tanycytes are a specialized cell type along the wall of the third ventricle2 that bidirectionally transport hormones and signalling molecules between the brain's parenchyma and ventricular system3-8. Here we show that tanycytes are activated upon acute thermal challenge and are necessary to reduce food intake afterwards. Virus-mediated gene manipulation and circuit mapping showed that thermosensing glutamatergic neurons of the parabrachial nucleus innervate tanycytes either directly or through second-order hypothalamic neurons. Heat-dependent Fos expression in tanycytes suggested their ability to produce signalling molecules, including vascular endothelial growth factor A (VEGFA). Instead of discharging VEGFA into the cerebrospinal fluid for a systemic effect, VEGFA was released along the parenchymal processes of tanycytes in the arcuate nucleus. VEGFA then increased the spike threshold of Flt1-expressing dopamine and agouti-related peptide (Agrp)-containing neurons, thus priming net anorexigenic output. Indeed, both acute heat and the chemogenetic activation of glutamatergic parabrachial neurons at thermoneutrality reduced food intake for hours, in a manner that is sensitive to both Vegfa loss-of-function and blockage of vesicle-associated membrane protein 2 (VAMP2)-dependent exocytosis from tanycytes. Overall, we define a multimodal neurocircuit in which tanycytes link parabrachial sensory relay to the long-term enforcement of a metabolic code.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures













References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

