Long-term in vivo three-photon imaging reveals region-specific differences in healthy and regenerative oligodendrogenesis
- PMID: 38539013
- PMCID: PMC11104262
- DOI: 10.1038/s41593-024-01613-7
Long-term in vivo three-photon imaging reveals region-specific differences in healthy and regenerative oligodendrogenesis
Abstract
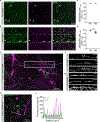
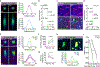
The generation of new myelin-forming oligodendrocytes in the adult central nervous system is critical for cognitive function and regeneration following injury. Oligodendrogenesis varies between gray and white matter regions, suggesting that local cues drive regional differences in myelination and the capacity for regeneration. However, the layer- and region-specific regulation of oligodendrocyte populations is unclear due to the inability to monitor deep brain structures in vivo. Here we harnessed the superior imaging depth of three-photon microscopy to permit long-term, longitudinal in vivo three-photon imaging of the entire cortical column and subcortical white matter in adult mice. We find that cortical oligodendrocyte populations expand at a higher rate in the adult brain than those of the white matter. Following demyelination, oligodendrocyte replacement is enhanced in the white matter, while the deep cortical layers show deficits in regenerative oligodendrogenesis and the restoration of transcriptional heterogeneity. Together, our findings demonstrate that regional microenvironments regulate oligodendrocyte population dynamics and heterogeneity in the healthy and diseased brain.
© 2024. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing Interests
KK is a co-founder and part-owner of 3i. The other authors declare no competing financial interests.
Figures

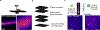
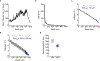








Update of
-
Long-term in vivo three-photon imaging reveals region-specific differences in healthy and regenerative oligodendrogenesis.bioRxiv [Preprint]. 2023 Nov 1:2023.10.29.564636. doi: 10.1101/2023.10.29.564636. bioRxiv. 2023. Update in: Nat Neurosci. 2024 May;27(5):846-861. doi: 10.1038/s41593-024-01613-7. PMID: 37961298 Free PMC article. Updated. Preprint.
References
-
- Flechsig Of Leipsic, P. DEVELOPMENTAL (MYELOGENETIC) LOCALISATION OF THE CEREBRAL CORTEX IN THE HUMAN SUBJECT. The Lancet 158, 1027–1030 (1901).
-
- Viganò F, Möbius W, Götz M & Dimou L Transplantation reveals regional differences in oligodendrocyte differentiation in the adult brain. Nat Neurosci 16, 1370–1372 (2013). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

