A whale of a tale: whale cells evade the driving mechanism for hexavalent chromium-induced chromosome instability
- PMID: 38539048
- PMCID: PMC11057468
- DOI: 10.1093/toxsci/kfae030
A whale of a tale: whale cells evade the driving mechanism for hexavalent chromium-induced chromosome instability
Abstract
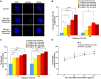
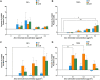
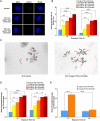
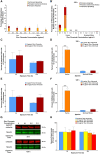
Chromosome instability, a hallmark of lung cancer, is a driving mechanism for hexavalent chromium [Cr(VI)] carcinogenesis in humans. Cr(VI) induces structural and numerical chromosome instability in human lung cells by inducing DNA double-strand breaks and inhibiting homologous recombination repair and causing spindle assembly checkpoint (SAC) bypass and centrosome amplification. Great whales are long-lived species with long-term exposures to Cr(VI) and accumulate Cr in their tissue, but exhibit a low incidence of cancer. Data show Cr(VI) induces fewer chromosome aberrations in whale cells after acute Cr(VI) exposure suggesting whale cells can evade Cr(VI)-induced chromosome instability. However, it is unknown if whales can evade Cr(VI)-induced chromosome instability. Thus, we tested the hypothesis that whale cells resist Cr(VI)-induced loss of homologous recombination repair activity and increased SAC bypass and centrosome amplification. We found Cr(VI) induces similar amounts of DNA double-strand breaks after acute (24 h) and prolonged (120 h) exposures in whale lung cells, but does not inhibit homologous recombination repair, SAC bypass, or centrosome amplification, and does not induce chromosome instability. These data indicate whale lung cells resist Cr(VI)-induced chromosome instability, the major driver for Cr(VI) carcinogenesis at a cellular level, consistent with observations that whales are resistant to cancer.
Keywords: DNA double-strand break; chromosome instability; hexavalent chromium; homologous recombination repair; whale.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- Brinkley B. R. (2001). Managing the centrosome numbers game: From chaos to stability in cancer cell division. Trends Cell Biol. 11, 18–21. - PubMed
-
- Bryant H. E., Ying S., Helleday T. (2006). Homologous recombination is involved in repair of chromium-induced DNA damage in mammalian cells. Mutat. Res. 599, 116–123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

