Comparative efficacy and safety of subcutaneous infliximab and vedolizumab in patients with Crohn's disease and ulcerative colitis included in randomised controlled trials
- PMID: 38539103
- PMCID: PMC10967176
- DOI: 10.1186/s12876-024-03163-5
Comparative efficacy and safety of subcutaneous infliximab and vedolizumab in patients with Crohn's disease and ulcerative colitis included in randomised controlled trials
Abstract
Background: While indirect comparison of infliximab (IFX) and vedolizumab (VDZ) in adults with Crohn's disease (CD) or ulcerative colitis (UC) shows that IFX has better effectiveness during induction, and comparable efficacy during maintenance treatment, comparative data specific to subcutaneous (SC) IFX (i.e., CT-P13 SC) versus VDZ are limited.
Aim: Pooled analysis of randomised studies to compare efficacy and safety with IFX SC and VDZ in moderate-to-severe inflammatory bowel disease.
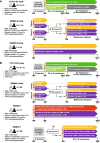
Methods: Parallel-group, randomised studies evaluating IFX SC and VDZ in patients with moderate-to-severe CD or UC were identified. Eligible studies reported ≥ 1 prespecified outcome of interest at Week 6 (reflecting treatment during the induction phase) and/or at 1 year (Weeks 50-54; reflecting treatment during the maintenance phase). Prespecified efficacy and safety outcomes considered in this pooled analysis included the proportions of patients achieving disease-specific clinical responses, clinical remission, or discontinuing due to lack of efficacy, and the proportions of patients experiencing adverse events (AEs), serious AEs, infections, serious infections, or discontinuing due to AEs. Data from multiple studies or study arms were extracted and pooled using a random-effect model; comparative analyses were performed separately for patients with CD and UC.
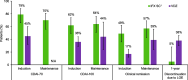
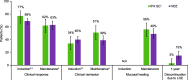
Results: We identified three eligible CD trials and four eligible UC trials that assigned over 1200 participants per disease cohort to either IFX SC or VDZ. In patients with CD, intravenous induction therapy with IFX demonstrated better efficacy (non-overlapping 95% confidence intervals [CIs]) compared with VDZ; during the maintenance phase, IFX SC showed numerically better efficacy (overlapping 95% CIs) than VDZ. A lower proportion of IFX SC-treated patients discontinued therapy due to lack of efficacy over 1 year. In patients with UC, efficacy profiles were similar with IFX SC and VDZ during the induction and maintenance phases, and a lower proportion of IFX SC-treated patients discontinued therapy due to lack of efficacy over 1 year. In both cohorts, safety profiles for IFX SC and VDZ were generally comparable during 1 year.
Conclusion: IFX SC demonstrated better efficacy than VDZ in patients with CD, and similar efficacy to VDZ in patients with UC; 1-year safety was comparable with IFX SC and VDZ.
Keywords: Biobetter; Bioinnovative; Inflammatory bowel disease; Subcutaneous infliximab; Tumour necrosis factor-α inhibitors; Vedolizumab.
© 2024. The Author(s).
Conflict of interest statement
Laurent Peyrin-Biroulet reports personal fees from AbbVie, Allergan, Alma, Amgen, Applied Molecular Transport, Arena, Biogen, BMS, Boehringer Ingelheim, Celgene, Celltrion, Enterome, Enthera, Ferring, Fresenius Kabi, Galapagos, Genentech, Gilead, Hikma, InDex Pharmaceuticals, Inotrem, Janssen, Lilly, MSD, Mylan, Nestle, Norgine, OSE Immunotherapeutics, Oppilan Pharma, Pfizer, Pharmacosmos, Roche, Samsung Bioepis, Sandoz, Sterna, Sublimity Therapeutics, Takeda, Theravance, Tillots, and Vifor. Perttu Arkkila has been an advisory board member of Janssen. Alessandro Armuzzi reports grants from Biogen, MSD, Pfizer, and Takeda; and personal fees from AbbVie, Allergan, Amgen, Arena, Biogen, Bristol Myers Squibb, Celltrion, Eli Lilly, Ferring, Galapagos, Gilead, Janssen, MSD, Mylan, Novartis, Pfizer, Protagonist Therapeutics, Roche, Samsung Bioepis, Sandoz, Takeda, and TiGenix. Silvio Danese reports personal fees from AbbVie, Allergan, Amgen, AstraZeneca, Athos Therapeutics, Biogen, Boehringer Ingelheim, Celgene, Celltrion, Eli Lilly, Enthera, Ferring Pharmaceuticals Inc., Gilead, Hospira, Inotrem, Janssen, Johnson & Johnson, MSD, Mundipharma, Mylan, Pfizer, Roche, Sandoz, Sublimity Therapeutics, Takeda, TiGenix, UCB Inc., and Vifor. Marc Ferrante reports grants from Janssen, Pfizer, Takeda, and Viatris; personal fees from AbbVie, Boehringer Ingelheim, Celgene, Eli Lilly, Falk, Janssen, Lamepro, Medtronic, Regeneron, Samsung Bioepis, Sandoz, Thermo Fisher, Truvion Healthcare, and Viatris. Jordi Guardiola reports personal fees from AbbVie, Chiesi, Ferring, GE Healthcare, Janssen, Kern Pharma, MSD, Pfizer, Roche, and Takeda. Jørgen Jahnsen reports personal fees from AbbVie, Astro Pharma, Boehringer Ingelheim, BMS, Celltrion, Ferring, Gilead, Hikma, Janssen, Meda, MSD, NappPharma, Novartis, Orion Pharma, Pfizer, Pharmacosmos, Roche, Sandoz, Takeda, Tillotts, and Unimedic Pharma. Edouard Louis has received research grants from Janssen, Pfizer, and Takeda; received educational grants from AbbVie, Janssen, and Takeda; received speaker fees from AbbVie, Celgene, Falk, Ferring, Janssen, MSD, Pfizer, and Takeda; been an advisory board member for AbbVie, Arena, Eli Lilly, Celgene, Ferring, Galapagos, Gilead, Janssen, MSD, Pfizer, and Takeda; and been a consultant of AbbVie. Milan Lukáš has received financial support for research and educational activities from Janssen, Pfizer, and Takeda; and has been an advisory board member for Egis, Janssen, and Takeda. Walter Reinisch reports grants from Abbott Laboratories, AbbVie, Aesca, Centocor, Falk, Immundiagnostik, Janssen, MSD, Sandoz, and Takeda. Xavier Roblin reports personal fees from Amgen, Celltrion, Gilead, Janssen, MSD, Pfizer, Takeda, and Tillots; personal fees from Abbott Laboratories, AbbVie, Aesca, Algernon, Amgen, AM Pharma, AMT, AOP Orphan, Aptalis, Arena Pharmaceuticals, Astellas, AstraZeneca, Avaxia, Bioclinica, Biogen IDEC, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Cellerix, Celltrion, Centocor, Chemocentryx, Covance, Danone Austria, DSM, Elan, Eli Lilly, Ernst & Young, Elan, Falk, Ferring, Galapagos, Gatehouse Bio Inc., Genentech, Gilead, Grünenthal, ICON, Immundiagnostik, InDex Pharmaceuticals, Inova, Intrinsic Imaging, J&J, Janssen, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, LivaNova, Mallinckrodt, Medahead, MedImmune, Millenium, Mitsubishi Tanabe Pharma Corporation, MSD, Nash Pharmaceuticals, Nestle, Nippon Kayaku, Novartis, Ocera, OMass, Otsuka, Parexel, PDL, Periconsulting, Pfizer, Pharmacosmos, Philip Morris Institute, PLS Education, Procter & Gamble, Prometheus, Protagonist, Provention, Quell Therapeutics, Robarts Clinical Trial, Roland Berger GmBH, Sandoz, Schering-Plough, Second Genome, Seres Therapeutics, Setpointmedical, Shire, Sigmoid, Sublimity, Takeda, Therakos, Theravance, TiGenix, UCB, Vifor, Yakult, Zeeland, Zyngenia, and 4SC. Philip J Smith reports personal fees from AbbVie, Celltrion, Dr Falk, Galapagos, Janssen, Takeda, and Tillotts Pharma. Taek Kwon, Jeeyoung Kim, Dong-Hyeon Kim, and Sangwook Yoon are employees of Celltrion. Raja Atreya reports grants and personal fees from AbbVie, Amgen, Arena Pharmaceuticals, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Celltrion, Falk Foundation, Ferring, Fresenius Kabi, Galapagos, Gilead, GlaxoSmithKline, InDex Pharmaceuticals, Janssen-Cilag, Kliniksa Pharmaceuticals, Lilly, Merck Sharp & Dohme, Novartis, Pandion, Pfizer, Roche Pharma, Samsung Bioepis, Takeda Pharma, and Viatris.
Figures
References
-
- Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, Hayee B, Lomer MCE, Parkes GC, Selinger C, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(Suppl 3):s1–s106. doi: 10.1136/gutjnl-2019-318484. - DOI - PMC - PubMed
-
- Burisch J, Kiudelis G, Kupcinskas L, Kievit HAL, Andersen KW, Andersen V, Salupere R, Pedersen N, Kjeldsen J, D'Incà R, et al. Natural disease course of Crohn's disease during the first 5 years after diagnosis in a European population-based inception cohort: an Epi-IBD study. Gut. 2019;68(3):423–433. doi: 10.1136/gutjnl-2017-315568. - DOI - PubMed

