Drug resistance in ovarian cancer: from mechanism to clinical trial
- PMID: 38539161
- PMCID: PMC10976737
- DOI: 10.1186/s12943-024-01967-3
Drug resistance in ovarian cancer: from mechanism to clinical trial
Abstract
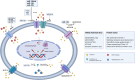
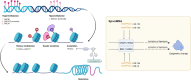
Ovarian cancer is the leading cause of gynecological cancer-related death. Drug resistance is the bottleneck in ovarian cancer treatment. The increasing use of novel drugs in clinical practice poses challenges for the treatment of drug-resistant ovarian cancer. Continuing to classify drug resistance according to drug type without understanding the underlying mechanisms is unsuitable for current clinical practice. We reviewed the literature regarding various drug resistance mechanisms in ovarian cancer and found that the main resistance mechanisms are as follows: abnormalities in transmembrane transport, alterations in DNA damage repair, dysregulation of cancer-associated signaling pathways, and epigenetic modifications. DNA methylation, histone modifications and noncoding RNA activity, three key classes of epigenetic modifications, constitute pivotal mechanisms of drug resistance. One drug can have multiple resistance mechanisms. Moreover, common chemotherapies and targeted drugs may have cross (overlapping) resistance mechanisms. MicroRNAs (miRNAs) can interfere with and thus regulate the abovementioned pathways. A subclass of miRNAs, "epi-miRNAs", can modulate epigenetic regulators to impact therapeutic responses. Thus, we also reviewed the regulatory influence of miRNAs on resistance mechanisms. Moreover, we summarized recent phase I/II clinical trials of novel drugs for ovarian cancer based on the abovementioned resistance mechanisms. A multitude of new therapies are under evaluation, and the preliminary results are encouraging. This review provides new insight into the classification of drug resistance mechanisms in ovarian cancer and may facilitate in the successful treatment of resistant ovarian cancer.
Keywords: Clinical trials; Ovarian cancer; Resistance mechanisms; miRNAs.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Ovarian Cancer — Cancer Stat Facts. [cited 2023 Aug 10]. Available from: https://seer.cancer.gov/statfacts/html/ovary.html.
-
- Ray-Coquard I, Leary A, Pignata S, Cropet C, González-Martin A, Marth C, et al. Olaparib plus bevacizumab first-line maintenance in ovarian cancer: final overall survival results from the PAOLA-1/ENGOT-ov25 trial. Ann Oncol Off J Eur Soc Med Oncol. 2023;S0923–7534(23):00686–695. - PubMed
-
- DiSilvestro P, Banerjee S, Colombo N, Scambia G, Kim B-G, Oaknin A, et al. Overall Survival With Maintenance Olaparib at a 7-Year Follow-Up in Patients With Newly Diagnosed Advanced Ovarian Cancer and a BRCA Mutation: The SOLO1/GOG 3004 Trial. J Clin Oncol Off J Am Soc Clin Oncol. 2023;41:609–617. doi: 10.1200/JCO.22.01549. - DOI - PMC - PubMed
-
- González-Martín A, Pothuri B, Vergote I, Graybill W, Lorusso D, McCormick CC, et al. Progression-free survival and safety at 3.5years of follow-up: results from the randomised phase 3 PRIMA/ENGOT-OV26/GOG-3012 trial of niraparib maintenance treatment in patients with newly diagnosed ovarian cancer. Eur J Cancer Oxf Engl. 1990;2023(189):112908. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- No. CSTB2023NSCQ-MSX1030, No. cstc2022jxjl120039/Chongqing Science and Technology Bureau
- No. CSTB2023NSCQ-MSX1030, No. cstc2022jxjl120039/Chongqing Science and Technology Bureau
- No. 2023ZDXM029, No. 2023MSXM043/Chongqing Health Commission
- No. 2023ZDXM029, No. 2023MSXM043/Chongqing Health Commission
- No. 82073129/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Medical

