Changes in lipid metabolism track with the progression of neurofibrillary pathology in tauopathies
- PMID: 38539208
- PMCID: PMC10976809
- DOI: 10.1186/s12974-024-03060-4
Changes in lipid metabolism track with the progression of neurofibrillary pathology in tauopathies
Abstract
Background: Accumulation of tau leads to neuroinflammation and neuronal cell death in tauopathies, including Alzheimer's disease. As the disease progresses, there is a decline in brain energy metabolism. However, the role of tau protein in regulating lipid metabolism remains less characterized and poorly understood.
Methods: We used a transgenic rat model for tauopathy to reveal metabolic alterations induced by neurofibrillary pathology. Transgenic rats express a tau fragment truncated at the N- and C-terminals. For phenotypic profiling, we performed targeted metabolomic and lipidomic analysis of brain tissue, CSF, and plasma, based on the LC-MS platform. To monitor disease progression, we employed samples from transgenic and control rats aged 4, 6, 8, 10, 12, and 14 months. To study neuron-glia interplay in lipidome changes induced by pathological tau we used well well-established multicomponent cell model system. Univariate and multivariate statistical approaches were used for data evaluation.
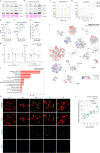
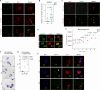
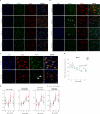
Results: We showed that tau has an important role in the deregulation of lipid metabolism. In the lipidomic study, pathological tau was associated with higher production of lipids participating in protein fibrillization, membrane reorganization, and inflammation. Interestingly, significant changes have been found in the early stages of tauopathy before the formation of high-molecular-weight tau aggregates and neurofibrillary pathology. Increased secretion of pathological tau protein in vivo and in vitro induced upregulated production of phospholipids and sphingolipids and accumulation of lipid droplets in microglia. We also found that this process depended on the amount of extracellular tau. During the later stages of tauopathy, we found a connection between the transition of tau into an insoluble fraction and changes in brain metabolism.
Conclusion: Our results revealed that lipid metabolism is significantly affected during different stages of tau pathology. Thus, our results demonstrate that the dysregulation of lipid composition by pathological tau disrupts the microenvironment, further contributing to the propagation of pathology.
Keywords: Lipid droplets; Lipidomics; Metabolomics; Microglia; Neurodegeneration; SHR24; Tau protein.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Iqbal K, del Alonso C, Chen A, Chohan S, El-Akkad MO, Gong E. C-X, Tau pathology in Alzheimer disease and other tauopathies. Biochimica et Biophysica Acta (BBA) - molecular basis of Disease. 2005;1739:198–210. - PubMed
-
- Cantrelle F-X, Loyens A, Trivelli X, Reimann O, Despres C, Gandhi NS et al. Phosphorylation and O-GlcNAcylation of the PHF-1 epitope of tau protein induce local conformational changes of the C-Terminus and modulate tau self-assembly into Fibrillar aggregates. Front Mol Neurosci. 2021;14. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

