Fetal and Infant Effects of Maternal Opioid Use during Pregnancy: A Literature Review including Clinical, Toxicological, Pharmacogenomic, and Epigenetic Aspects for Forensic Evaluation
- PMID: 38539313
- PMCID: PMC10969201
- DOI: 10.3390/children11030278
Fetal and Infant Effects of Maternal Opioid Use during Pregnancy: A Literature Review including Clinical, Toxicological, Pharmacogenomic, and Epigenetic Aspects for Forensic Evaluation
Abstract
The two primary classes of opioid substances are morphine and its synthetic derivative, heroin. Opioids can cross the placental barrier, reaching fetal circulation. Therefore, at any gestational age, the fetus is highly exposed to pharmacologically active opioid metabolites and their associated adverse effects. This review aimed to investigate all the studies reported in a timeframe of forty years about prenatal and postnatal outcomes of opioid exposition during pregnancy. Clinical and toxicological aspects, as well as pharmacogenetic and epigenetic research focusing on fetal and infant effects of opioid use during pregnancy together with their medico-legal implications are exposed and discussed.
Keywords: buprenorphine; epigenetic; methadone; neonatal withdrawal symptoms from opioid drugs (NOWS); opioids in pregnancy; pharmacogenomic; toxicology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

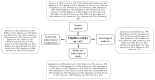
References
-
- National Institute on Drug Abuse, Substance Use While Pregnant and Breast Feeding. [(accessed on 27 December 2022)]; Available online: https://nida.nih.gov/publications/research-reports/substance-use-in-wome....
-
- Substance Abuse and Mental Health Services Administration 2020 National Survey on Drug Use and Health: Detailed Tables. [(accessed on 27 December 2022)]; Available online: https://www.samhsa.gov/data/report/2020-nsduh-detailed-tables.
-
- Bertaso A., Gottardo R., Murari M., Mazzola M., Porpiglia N.M., Taus F., Beghini R., Gandini F., Bortolotti F. Hair testing applied to the assessment of in utero exposure to drugs: Critical analysis of 51 cases of the University Hospital of Verona. Drug Test. Anal. 2023;15:980–986. doi: 10.1002/dta.3515. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

