De-Escalation Surgery in cT3-4 Breast Cancer Patients after Neoadjuvant Therapy: Predictors of Breast Conservation and Comparison of Long-Term Oncological Outcomes with Mastectomy
- PMID: 38539504
- PMCID: PMC10969431
- DOI: 10.3390/cancers16061169
De-Escalation Surgery in cT3-4 Breast Cancer Patients after Neoadjuvant Therapy: Predictors of Breast Conservation and Comparison of Long-Term Oncological Outcomes with Mastectomy
Abstract
Background: Neoadjuvant therapy (NAT) has become increasingly employed for the treatment of cT3-4 breast cancer (BC), enabling breast-conserving surgery (BCS) in cases traditionally considered for mastectomy. This study aims to identify predictors for breast conservation post-NAT and to evaluate whether BCS influences long-term oncological outcomes.
Methods: We retrospectively analyzed data from patients with cT3-4 BC who received NAT at the Breast Unit of IRCCS Humanitas Research Hospital, Milan, Italy, from October 2009 to April 2020. Surgical outcomes and long-term oncological results, such as disease-free survival (DFS), distant DFS (DDFS), overall survival (OS), and BC-specific survival (BCSS), were compared between the BCS and mastectomy groups.
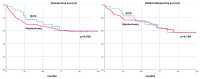
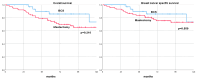
Results: Among 114 patients analyzed, 37 (32.5%) underwent BCS, and 77 (67.5%) had a mastectomy. The key predictors for opting for BCS included absence of vascular invasion, reduced tumor size post-NAT, and achieving ypT0 status. No significant differences in DFS, DDFS, OS, and BCSS were observed between the two surgical groups (log-ranks, p = 0.520, p = 0.789, p = 0.216, p = 0.559, respectively).
Conclusions: BCS after NAT is a feasible and safe option for patients with cT3-4 BC, without adversely affecting long-term oncological outcomes. Identifying predictors of breast conservation can guide surgical decision-making, ensuring that patients receive optimal treatment.
Keywords: breast cancer; breast-conserving surgery; de-escalation; mastectomy; neoadjuvant therapy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Choudhary P., Gogia A., Deo S., Mathur S., Sharma D. Neoadjuvant Chemotherapy in Locally Advanced Breast Cancer: Clinicopathological Characteristics and Correlation with Pathological Complete Response. J. Clin. Oncol. 2020;38:e12658. doi: 10.1200/JCO.2020.38.15_suppl.e12658. - DOI
-
- Hortobagyi G.N., Ames F.C., Buzdar A.U., Kau S.W., McNeese M.D., Paulus D., Hug V., Holmes F.A., Romsdahl M.M., Fraschini G., et al. Management of Stage III Primary Breast Cancer with Primary Chemotherapy, Surgery, and Radiation Therapy. Cancer. 1988;62:2507–2516. doi: 10.1002/1097-0142(19881215)62:12<2507::AID-CNCR2820621210>3.0.CO;2-D. - DOI - PubMed
-
- Cortazar P., Zhang L., Untch M., Mehta K., Costantino J.P., Wolmark N., Bonnefoi H., Cameron D., Gianni L., Valagussa P., et al. Pathological Complete Response and Long-Term Clinical Benefit in Breast Cancer: The CTNeoBC Pooled Analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

