Current Treatment Options for Renal Cell Carcinoma: Focus on Cell-Based Immunotherapy
- PMID: 38539542
- PMCID: PMC10968958
- DOI: 10.3390/cancers16061209
Current Treatment Options for Renal Cell Carcinoma: Focus on Cell-Based Immunotherapy
Abstract
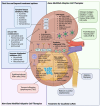
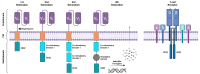
Renal cell carcinoma (RCC) affects over 400,000 patients globally each year, and 30% of patients present with metastatic disease. Current standard of care therapy for metastatic RCC involve TKIs and ICIs, including combinatorial strategies, but this offers only modest clinical benefit. Novel treatment approaches are warranted, and cell-based immunotherapies for RCC hold significant promise. These are currently being tested in the pre-clinical setting and in early phase clinical trials. Here, we review the landscape of cellular immunotherapy for RCC in the context of currently available therapies, with a particular focus on defining the current best antigenic targets, the range of cell therapy products being explored in RCC, and how advanced engineering solutions may further enhance these therapies in the RCC space.
Keywords: chimeric antigen receptor (CAR) T-cells; engineered TCR T-cells; immunotherapy; renal cell carcinoma.
Conflict of interest statement
M.T. has received speaker and consultancy fees from MSD, Angiodynamics and Boston Scientific. The remaining authors declare no relevant conflicts of interest.
Figures
Similar articles
-
Novel Cellular Therapies for Hepatocellular Carcinoma.Cancers (Basel). 2022 Jan 20;14(3):504. doi: 10.3390/cancers14030504. Cancers (Basel). 2022. PMID: 35158772 Free PMC article. Review.
-
Current and future perspectives on CAR-T cell therapy for renal cell carcinoma: A comprehensive review.Investig Clin Urol. 2022 Sep;63(5):486-498. doi: 10.4111/icu.20220103. Investig Clin Urol. 2022. PMID: 36067994 Free PMC article. Review.
-
Future perspectives in melanoma research : Meeting report from the "Melanoma Bridge". Napoli, December 1st-4th 2015.J Transl Med. 2016 Nov 15;14(1):313. doi: 10.1186/s12967-016-1070-y. J Transl Med. 2016. PMID: 27846884 Free PMC article.
-
Systemic Treatment of Metastatic Clear Cell Renal Cell Carcinoma in 2018: Current Paradigms, Use of Immunotherapy, and Future Directions.Eur Urol. 2019 Jan;75(1):100-110. doi: 10.1016/j.eururo.2018.10.010. Epub 2018 Oct 13. Eur Urol. 2019. PMID: 30327274 Review.
-
Paediatric Strategy Forum for medicinal product development of chimeric antigen receptor T-cells in children and adolescents with cancer: ACCELERATE in collaboration with the European Medicines Agency with participation of the Food and Drug Administration.Eur J Cancer. 2022 Jan;160:112-133. doi: 10.1016/j.ejca.2021.10.016. Epub 2021 Nov 25. Eur J Cancer. 2022. PMID: 34840026 Review.
Cited by
-
Alternative Splicing in Tumorigenesis and Cancer Therapy.Biomolecules. 2025 May 29;15(6):789. doi: 10.3390/biom15060789. Biomolecules. 2025. PMID: 40563429 Free PMC article. Review.
-
Targeting Sodium Transport Reveals CHP1 Downregulation as a Novel Molecular Feature of Malignant Progression in Clear Cell Renal Cell Carcinoma: Insights from Integrated Multi-Omics Analyses.Biomolecules. 2025 Jul 15;15(7):1019. doi: 10.3390/biom15071019. Biomolecules. 2025. PMID: 40723891 Free PMC article.
-
Integrative multi-omics reveal NSUN2 facilitates glycolysis and histone lactylation-driven immune evasion in renal carcinoma.Genes Immun. 2025 Aug;26(4):312-323. doi: 10.1038/s41435-025-00336-4. Epub 2025 May 24. Genes Immun. 2025. PMID: 40413354
References
-
- Kidney Cancer Incidence Statistics. [(accessed on 5 December 2023)]. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/s....
-
- Cancer of the Kidney and Renal Pelvis—Cancer Stat Facts. [(accessed on 10 January 2024)]; Available online: https://seer.cancer.gov/statfacts/html/kidrp.html.
Publication types
LinkOut - more resources
Full Text Sources

