Reactive Oxygen Species Signaling and Oxidative Stress: Transcriptional Regulation and Evolution
- PMID: 38539845
- PMCID: PMC10967436
- DOI: 10.3390/antiox13030312
Reactive Oxygen Species Signaling and Oxidative Stress: Transcriptional Regulation and Evolution
Abstract
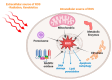
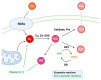
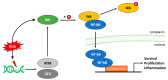
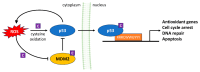
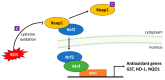
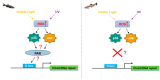
Since the evolution of the aerobic metabolism, reactive oxygen species (ROS) have represented significant challenges to diverse life forms. In recent decades, increasing knowledge has revealed a dual role for ROS in cell physiology, showing they serve as a major source of cellular damage while also functioning as important signaling molecules in various biological processes. Our understanding of ROS homeostasis and ROS-mediated cellular signaling pathways has presumed that they are ancient and highly conserved mechanisms shared by most organisms. However, emerging evidence highlights the complexity and plasticity of ROS signaling, particularly in animals that have evolved in extreme environments. In this review, we focus on ROS generation, antioxidative systems and the main signaling pathways that are influenced by ROS. In addition, we discuss ROS's responsive transcription regulation and how it may have been shaped over the course of evolution.
Keywords: DNA repair; cellular signaling; reactive oxygen species; transcriptional regulation; vertebrate evolution.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

