Cyanide Insensitive Oxidase Confers Hydrogen Sulfide and Nitric Oxide Tolerance to Pseudomonas aeruginosa Aerobic Respiration
- PMID: 38539916
- PMCID: PMC10968556
- DOI: 10.3390/antiox13030383
Cyanide Insensitive Oxidase Confers Hydrogen Sulfide and Nitric Oxide Tolerance to Pseudomonas aeruginosa Aerobic Respiration
Abstract
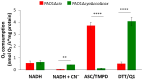
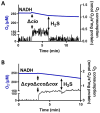
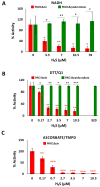
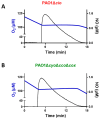
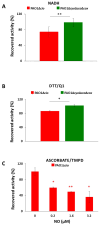
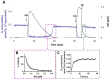
Hydrogen sulfide (H2S) and nitric oxide (NO) are long-known inhibitors of terminal oxidases in the respiratory chain. Yet, they exert pivotal signaling roles in physiological processes, and in several bacterial pathogens have been reported to confer resistance against oxidative stress, host immune responses, and antibiotics. Pseudomonas aeruginosa, an opportunistic pathogen causing life-threatening infections that are difficult to eradicate, has a highly branched respiratory chain including four terminal oxidases of the haem-copper type (aa3, cbb3-1, cbb3-2, and bo3) and one oxidase of the bd-type (cyanide-insensitive oxidase, CIO). As Escherichia coli bd-type oxidases have been shown to be H2S-insensitive and to readily recover their activity from NO inhibition, here we tested the effect of H2S and NO on CIO by performing oxygraphic measurements on membrane preparations from P. aeruginosa PAO1 and isogenic mutants depleted of CIO only or all other terminal oxidases except CIO. We show that O2 consumption by CIO is unaltered even in the presence of high levels of H2S, and that CIO expression is enhanced and supports bacterial growth under such stressful conditions. In addition, we report that CIO is reversibly inhibited by NO, while activity recovery after NO exhaustion is full and fast, suggesting a protective role of CIO under NO stress conditions. As P. aeruginosa is exposed to H2S and NO during infection, the tolerance of CIO towards these stressors agrees with the proposed role of CIO in P. aeruginosa virulence.
Keywords: Pseudomonas aeruginosa; bd-type terminal oxidases; cyanide insensitive oxidase; hydrogen sulfide; nitric oxide; respiratory chain.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
A cytochrome bd repressed by a MarR family regulator confers resistance to metals, nitric oxide, sulfide, and cyanide in Chromobacterium violaceum.Appl Environ Microbiol. 2025 Feb 19;91(2):e0236024. doi: 10.1128/aem.02360-24. Epub 2025 Jan 24. Appl Environ Microbiol. 2025. PMID: 39853125 Free PMC article.
-
Differential expression of multiple terminal oxidases for aerobic respiration in Pseudomonas aeruginosa.Environ Microbiol. 2010 Jun;12(6):1399-412. doi: 10.1111/j.1462-2920.2009.02109.x. Epub 2009 Nov 23. Environ Microbiol. 2010. PMID: 19930444
-
A cytochrome bd repressed by a MarR family regulator confers resistance to metals, nitric oxide, sulfide, and cyanide in Chromobacterium violaceum.bioRxiv [Preprint]. 2024 Aug 7:2024.08.06.606881. doi: 10.1101/2024.08.06.606881. bioRxiv. 2024. Update in: Appl Environ Microbiol. 2025 Feb 19;91(2):e0236024. doi: 10.1128/aem.02360-24. PMID: 39211195 Free PMC article. Updated. Preprint.
-
Impact of Hydrogen Sulfide on Mitochondrial and Bacterial Bioenergetics.Int J Mol Sci. 2021 Nov 24;22(23):12688. doi: 10.3390/ijms222312688. Int J Mol Sci. 2021. PMID: 34884491 Free PMC article. Review.
-
Terminal Oxidase Cytochrome bd Protects Bacteria Against Hydrogen Sulfide Toxicity.Biochemistry (Mosc). 2021 Jan;86(1):22-32. doi: 10.1134/S000629792101003X. Biochemistry (Mosc). 2021. PMID: 33705279 Review.
Cited by
-
Carbon Monoxide and Prokaryotic Energy Metabolism.Int J Mol Sci. 2025 Mar 20;26(6):2809. doi: 10.3390/ijms26062809. Int J Mol Sci. 2025. PMID: 40141451 Free PMC article. Review.
References
-
- Deshmukh R., Harwansh R.K., Bandyopadhyay N., Bandopadhyay S., Kumar P. Frontiers in Pharmacology of Neurotransmitters. Springer; Singapore: 2020. Pharmacology of Gasotransmitters (Nitric Oxide and Carbon Monoxide) and Their Action; pp. 579–617.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

