Comparative EPR Studies on the Influence of Genistein on Free Radicals in Non-Irradiated and UV-Irradiated MCF7, T47D and MDA-MB-231 Breast Cancer Cells
- PMID: 38540131
- PMCID: PMC10967802
- DOI: 10.3390/biomedicines12030518
Comparative EPR Studies on the Influence of Genistein on Free Radicals in Non-Irradiated and UV-Irradiated MCF7, T47D and MDA-MB-231 Breast Cancer Cells
Abstract
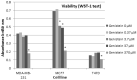
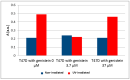
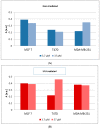
The antioxidant activity and the association of genistein with carcinogenesis are widely documented. Few studies directly measure the number of free radicals generated in cells, either during the action of factors stimulating their formation, e.g., ultraviolet (UV), or after exposure to antioxidants. The most suitable method for analysing free radicals is electron paramagnetic resonance (EPR) spectroscopy. The EPR method detects a paramagnetic centre with a single electron. Antioxidants neutralize free radicals, therefore, EPR analysis of antioxidant efficacy is as valuable and important as studying the paramagnetic centres of radicals. The aim of the study was to determine the influence of genistein on free radicals basal level and after UV exposure in breast cancer cell lines MCF7, T47D and MDA-MB-231 cell lines. The impact of genistein on cell viability was investigated at concentrations of 0.37 μM, 3.7 μM, 37 μM and 370 μM. Genistein at a concentration of 370 μM revealed a cytotoxic effect on the cells of all three tested breast cancer lines. Genistein at a concentration of 0.37 μM showed no significant effect on the cell viability of all tested breast cancer lines. Therefore, cell proliferation and antioxidant properties were examined using genistein at a concentration of 0.37 μM and 37 μM. X-band (9.3 GHz) EPR spectra of three different types of breast cancer cells (ER-positive, PR-positive and HER-2 negative: MCF7 and T47D and triple-negative MDA-MB-231) were compared. UV irradiation was used as a factor to generate free radicals in cells. The effect of free radical interactions with the antioxidant genistein was tested for non-UV-irradiated (corresponding to the basal level of free radicals in cells) and UV-irradiated cells. The levels of free radicals in the non-irradiated cells studied increased in the following order in breast cancer cells: T47D < MDA-MB-231 < MCF7 and UV-irradiated breast cancer cells: MDA-MB-231 < MCF7 < T47D. UV-irradiation altered free radical levels in all control and genistein-cultured cells tested. UV irradiation caused a slight decrease in the amount of free radicals in MCF7 cells. A strong decrease in the amount of free radicals was observed in UV-irradiated MDA-MB-231 breast cancer cells. The amount of free radicals in T47D cancer cells increased after UV irradiation. Genistein decreased the amount of free radicals in non-irradiated and UV-irradiated MCF7 cells, and only a weak effect of genistein concentrations was reported. Genistein greatly decreased the amount of free radicals in UV-irradiated T47D cancer cells cultured with genistein at a concentration of 3.7 μM. The effect of genistein was negligible in the other samples. Genistein at a concentration of 3.7 μM decreased the amount of free radicals in non-irradiated MDA-MB-231 cancer cells, but genistein at a concentration of 37 μM did not change the amount of free radicals in these cells. An increase in the amount of free radicals in UV-irradiated MDA-MB-231 cancer cells was observed with increasing genistein concentration. The antioxidant efficacy of genistein as a potential plant-derived agent supporting the treatment of various cancers may be determined by differences in signalling pathways that are characteristic of breast cancer cell line subtypes and differences in activation of oxidative stress response pathways.
Keywords: EPR spectroscopy; MDA-MB-231); T47D; breast cancer cells (MCF7; free radicals; genistein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

