Amelioration of Morphological Pathology in Cardiac, Respiratory, and Skeletal Muscles Following Intraosseous Administration of Human Dystrophin Expressing Chimeric (DEC) Cells in Duchenne Muscular Dystrophy Model
- PMID: 38540201
- PMCID: PMC10968566
- DOI: 10.3390/biomedicines12030586
Amelioration of Morphological Pathology in Cardiac, Respiratory, and Skeletal Muscles Following Intraosseous Administration of Human Dystrophin Expressing Chimeric (DEC) Cells in Duchenne Muscular Dystrophy Model
Abstract
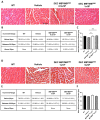
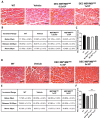
Duchenne Muscular Dystrophy (DMD) is a lethal disease caused by mutation in the dystrophin gene. Currently there is no cure for DMD. We introduced a novel human Dystrophin Expressing Chimeric (DEC) cell therapy of myoblast origin and confirmed the safety and efficacy of DEC in the mdx mouse models of DMD. In this study, we assessed histological and morphological changes in the cardiac, diaphragm, and gastrocnemius muscles of the mdx/scid mice after the transplantation of human DEC therapy via the systemic-intraosseous route. The efficacy of different DEC doses was evaluated at 90 days (0.5 × 106 and 1 × 106 DEC cells) and 180 days (1 × 106 and 5 × 106 DEC cells) after administration. The evaluation of Hematoxylin & Eosin (H&E)-stained sectional slices of cardiac, diaphragm, and gastrocnemius muscles included assessment of muscle fiber size by minimal Feret's diameter method using ImageJ software. The overall improvement in muscle morphology was observed in DMD-affected target muscles in both studies, as evidenced by a shift in fiber size distribution toward the wild type (WT) phenotype and by an increase in the mean Feret's diameter compared to the vehicle-injected controls. These findings confirm the long-term efficacy of human DEC therapy in the improvement of overall morphological pathology in the muscles affected by DMD and introduce DEC as a novel therapeutic approach for DMD patients.
Keywords: DEC therapy; Duchenne Muscular Dystrophy (DMD); Dystrophin Expressing Chimeric (DEC) cells; cardiac and skeletal muscle morphology; mdx mice; minimal Feret’s fiber diameter; muscle fibrosis; stem cells; systemic-intraosseous administration.
Conflict of interest statement
M.S. is CMO and shareholder of Dystrogen Therapeutics SA, the company that holds a license for DEC Therapy. The author declares a potential financial conflict of interest. M.S. is the inventor on the patent application filed by the University of Illinois at Chicago related to chimeric cell therapy for Duchenne Muscular Dystrophy (WO/2016/201182). K.S. is CEO and shareholder of Dystrogen Therapeutics Corp. A.H. is the adviser to Dystrogen Therapeutics Corp. The authors declare a potential financial conflict of interest. The authors M.S., K.B., K.Z., P.L., S.B., K.S. and A.H. do not have any non-financial conflicts of interest.
Figures


References
-
- Van Ruiten H.J., Bettolo C.M., Cheetham T., Eagle M., Lochmuller H., Straub V., Bushby K., Guglieri M. Why are some patients with Duchenne muscular dystrophy dying young: An analysis of causes of death in North East England. Eur. J. Paediatr. Neurol. 2016;20:904–909. doi: 10.1016/j.ejpn.2016.07.020. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

