The Impact of Microbiota-Immunity-Hormone Interactions on Autoimmune Diseases and Infection
- PMID: 38540229
- PMCID: PMC10967803
- DOI: 10.3390/biomedicines12030616
The Impact of Microbiota-Immunity-Hormone Interactions on Autoimmune Diseases and Infection
Abstract
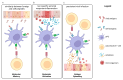
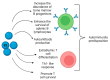
Autoimmune diseases are complex multifactorial disorders, and a mixture of genetic and environmental factors play a role in their onset. In recent years, the microbiota has gained attention as it helps to maintain host health and immune homeostasis and is a relevant player in the interaction between our body and the outside world. Alterations (dysbiosis) in its composition or function have been linked to different pathologies, including autoimmune diseases. Among the different microbiota functions, there is the activation/modulation of immune cells that can protect against infections. However, if dysbiosis occurs, it can compromise the host's ability to protect against pathogens, contributing to the development and progression of autoimmune diseases. In some cases, infections can trigger autoimmune diseases by several mechanisms, including the alteration of gut permeability and the activation of innate immune cells to produce pro-inflammatory cytokines that recruit autoreactive T and B cells. In this complex scenario, we cannot neglect critical hormones' roles in regulating immune responses. Different hormones, especially estrogens, have been shown to influence the development and progression of autoimmune diseases by modulating the activity and function of the immune system in different ways. In this review, we summarized the main mechanisms of connection between infections, microbiota, immunity, and hormones in autoimmune diseases' onset and progression given the influence of some infections and hormone levels on their pathogenesis. In detail, we focused on rheumatoid arthritis, multiple sclerosis, and systemic lupus erythematosus.
Keywords: autoimmune diseases; hormones; immune system; infections; microbiota.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

