Immune Analysis Using Vitreous Optical Coherence Tomography Imaging in Rats with Steroid-Induced Glaucoma
- PMID: 38540246
- PMCID: PMC10967960
- DOI: 10.3390/biomedicines12030633
Immune Analysis Using Vitreous Optical Coherence Tomography Imaging in Rats with Steroid-Induced Glaucoma
Abstract
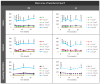
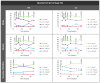
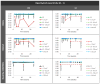
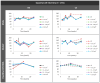
Glaucoma is a multifactorial pathology involving the immune system. The subclinical immune response plays a homeostatic role in healthy situations, but in pathological situations, it produces imbalances. Optical coherence tomography detects immune cells in the vitreous as hyperreflective opacities and these are subsequently characterised by computational analysis. This study monitors the changes in immunity in the vitreous in two steroid-induced glaucoma (SIG) animal models created with drug delivery systems (microspheres loaded with dexamethasone and dexamethasone/fibronectin), comparing both sexes and healthy controls over six months. SIG eyes tended to present greater intensity and a higher number of vitreous opacities (p < 0.05), with dynamic fluctuations in the percentage of isolated cells (10 µm2), non-activated cells (10-50 µm2), activated cells (50-250 µm2) and cell complexes (>250 µm2). Both SIG models presented an anti-inflammatory profile, with non-activated cells being the largest population in this study. However, smaller opacities (isolated cells) seemed to be the first responder to noxa since they were the most rounded (recruitment), coinciding with peak intraocular pressure increase, and showed the highest mean Intensity (intracellular machinery), even in the contralateral eye, and a major change in orientation (motility). Studying the features of hyperreflective opacities in the vitreous using OCT could be a useful biomarker of glaucoma.
Keywords: animal models; glaucoma; inflammation; optical coherence tomography; vitreous body.
Conflict of interest statement
Authors L.E.P. and M.S. were employed by the company Biotech Vision SLP (Spin-Off Company). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





References
Grants and funding
- Grants M17/00213, JR22/00057, PI17/01726, PI17/01946 and PI20/00437/Instituto de Salud Carlos III
- MAT2017-83858-C2-1, MAT2017-83858-C2-2, MCIN/AEI/ 10.13039/501100011033. PID2020-113281RB-C2-1 and PID2020-113281RB-C2-2, and co-funded by the European Union ERDR A way of making Europe/Ministerio de Asuntos Económicos y Transformación Digital
LinkOut - more resources
Full Text Sources

