Menthol Pretreatment Alleviates Campylobacter jejuni-Induced Enterocolitis in Human Gut Microbiota-Associated IL-10-/- Mice
- PMID: 38540710
- PMCID: PMC10968592
- DOI: 10.3390/biom14030290
Menthol Pretreatment Alleviates Campylobacter jejuni-Induced Enterocolitis in Human Gut Microbiota-Associated IL-10-/- Mice
Abstract
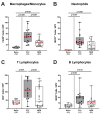
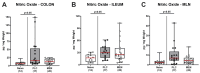
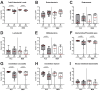
Human Campylobacter jejuni infections are of worldwide importance and represent the most commonly reported bacterial enteritis cases in middle- and high-income countries. Since antibiotics are usually not indicated and the severity of campylobacteriosis is directly linked to the risk of developing post-infectious complications, non-toxic antibiotic-independent treatment approaches are highly desirable. Given its health-promoting properties, including anti-microbial and anti-inflammatory activities, we tested the disease-alleviating effects of oral menthol in murine campylobacteriosis. Therefore, human gut microbiota-associated IL-10-/- mice were orally subjected to synthetic menthol starting a week before C. jejuni infection and followed up until day 6 post-infection. Whereas menthol pretreatment did not improve campylobacteriosis symptoms, it resulted in reduced colonic C. jejuni numbers and alleviated both macroscopic and microscopic aspects of C. jejuni infection in pretreated mice vs. controls. Menthol pretreatment dampened the recruitment of macrophages, monocytes, and T lymphocytes to colonic sites of infection, which was accompanied by mitigated intestinal nitric oxide secretion. Furthermore, menthol pretreatment had only marginal effects on the human fecal gut microbiota composition during the C. jejuni infection. In conclusion, the results of this preclinical placebo-controlled intervention study provide evidence that menthol application constitutes a promising way to tackle acute campylobacteriosis, thereby reducing the risk for post-infectious complications.
Keywords: Campylobacter jejuni; anti-oxidant effects; campylobacteriosis model; host-pathogen interaction; human gut microbiota-associated IL-10−/− mice; immune-modulatory properties; menthol; placebo-controlled preclinical intervention study; pretreatment.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Oral curcumin ameliorates acute murine campylobacteriosis.Front Immunol. 2024 May 24;15:1363457. doi: 10.3389/fimmu.2024.1363457. eCollection 2024. Front Immunol. 2024. PMID: 38855111 Free PMC article.
-
Prophylactic Oral Application of Activated Charcoal Mitigates Acute Campylobacteriosis in Human Gut Microbiota-Associated IL-10-/- Mice.Biomolecules. 2024 Jan 23;14(2):141. doi: 10.3390/biom14020141. Biomolecules. 2024. PMID: 38397378 Free PMC article.
-
Murine Fecal Microbiota Transplantation Alleviates Intestinal and Systemic Immune Responses in Campylobacter jejuni Infected Mice Harboring a Human Gut Microbiota.Front Immunol. 2019 Sep 24;10:2272. doi: 10.3389/fimmu.2019.02272. eCollection 2019. Front Immunol. 2019. PMID: 31616437 Free PMC article.
-
Re-thinking the chicken-Campylobacter jejuni interaction: a review.Avian Pathol. 2018 Aug;47(4):352-363. doi: 10.1080/03079457.2018.1475724. Epub 2018 Jun 11. Avian Pathol. 2018. PMID: 29764197 Review.
-
Novel Clinical Campylobacter jejuni Infection Models Based on Sensitization of Mice to Lipooligosaccharide, a Major Bacterial Factor Triggering Innate Immune Responses in Human Campylobacteriosis.Microorganisms. 2020 Mar 28;8(4):482. doi: 10.3390/microorganisms8040482. Microorganisms. 2020. PMID: 32231139 Free PMC article. Review.
Cited by
-
Therapeutic effects of oral benzoic acid application during acute murine campylobacteriosis.Eur J Microbiol Immunol (Bp). 2024 May 27;14(3):243-260. doi: 10.1556/1886.2024.00059. Print 2024 Sep 11. Eur J Microbiol Immunol (Bp). 2024. PMID: 38801662 Free PMC article.
-
Therapeutic and protective approaches to combat Campylobacter jejuni infections.Front Pharmacol. 2025 May 6;16:1572616. doi: 10.3389/fphar.2025.1572616. eCollection 2025. Front Pharmacol. 2025. PMID: 40395728 Free PMC article. Review.
References
-
- Oyarzabal O.A., Backert S. Microbial Food Safety. Springer; Berlin/Heidelberg, Germany: 2012. Food Safety Resources; pp. 235–239.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

