An Engineered Laccase from Fomitiporia mediterranea Accelerates Lignocellulose Degradation
- PMID: 38540744
- PMCID: PMC10968408
- DOI: 10.3390/biom14030324
An Engineered Laccase from Fomitiporia mediterranea Accelerates Lignocellulose Degradation
Abstract
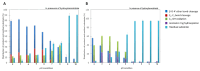
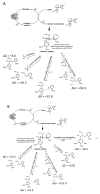
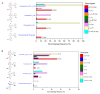
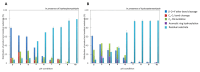
Laccases from white-rot fungi catalyze lignin depolymerization, a critical first step to upgrading lignin to valuable biodiesel fuels and chemicals. In this study, a wildtype laccase from the basidiomycete Fomitiporia mediterranea (Fom_lac) and a variant engineered to have a carbohydrate-binding module (Fom_CBM) were studied for their ability to catalyze cleavage of β-O-4' ether and C-C bonds in phenolic and non-phenolic lignin dimers using a nanostructure-initiator mass spectrometry-based assay. Fom_lac and Fom_CBM catalyze β-O-4' ether and C-C bond breaking, with higher activity under acidic conditions (pH < 6). The potential of Fom_lac and Fom_CBM to enhance saccharification yields from untreated and ionic liquid pretreated pine was also investigated. Adding Fom_CBM to mixtures of cellulases and hemicellulases improved sugar yields by 140% on untreated pine and 32% on cholinium lysinate pretreated pine when compared to the inclusion of Fom_lac to the same mixtures. Adding either Fom_lac or Fom_CBM to mixtures of cellulases and hemicellulases effectively accelerates enzymatic hydrolysis, demonstrating its potential applications for lignocellulose valorization. We postulate that additional increases in sugar yields for the Fom_CBM enzyme mixtures were due to Fom_CBM being brought more proximal to lignin through binding to either cellulose or lignin itself.
Keywords: Fomitiporia mediterranea; Komagataella pastoris expression; laccase; lignin; nanostructure-initiator mass spectrometry (NIMS).
Conflict of interest statement
B.A.S. has a financial interest in Illium Technologies, Caribou Biofuels, and Erg Bio. All other authors declare the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures