Postweaning Development Influences Endogenous VPAC1 Modulation of LTP Induced by Theta-Burst Stimulation: A Link to Maturation of the Hippocampal GABAergic System
- PMID: 38540797
- PMCID: PMC10968312
- DOI: 10.3390/biom14030379
Postweaning Development Influences Endogenous VPAC1 Modulation of LTP Induced by Theta-Burst Stimulation: A Link to Maturation of the Hippocampal GABAergic System
Abstract
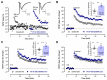
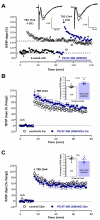
Long-term potentiation (LTP) induced by theta-burst stimulation (TBS) undergoes postweaning developmental changes partially linked to GABAergic circuit maturation. Endogenous vasoactive intestinal peptide (VIP) acting on its VPAC1 receptor strongly influences LTP induced by theta-burst stimulation (TBS), an effect dependent on GABAergic transmission. Although VPAC1 receptor levels are developmentally regulated during embryogenesis, their variation along postweaning development is unknown, as is the VPAC1 modulation of LTP or its relation to hippocampal GABAergic circuit maturation. As such, we investigated how VPAC1 modulation of LTP adjusts from weaning to adulthood along with GABAergic circuit maturation. As described, LTP induced by mild TBS (5 bursts, 4 pulses delivered at 100 Hz) was increasingly greater from weaning to adulthood. The influence of the VPAC1 receptor antagonist PG 97-269 (100 nM) on TBS-induced LTP was much larger in juvenile (3-week-old) than in young adult (6-7-week-old) or adult (12-week-old) rats. This effect was not associated with a developmental decrease in synaptic VPAC1 receptor levels. However, an increase in pre and post-synaptic GABAergic synaptic markers suggests an increase in the number of GABAergic synaptic contacts that is more prominent than the one observed in glutamatergic connections during this period. Conversely, endogenous VPAC2 receptor activation did not significantly influence TBS-induced LTP. VPAC2 receptor levels enhance pronouncedly during postweaning development, but not at synaptic sites. Given the involvement of VIP interneurons in several aspects of hippocampal-dependent learning, neurodevelopmental disorders, and epilepsy, this could provide important insights into the role of VIP modulation of hippocampal synaptic plasticity during normal and altered brain development potentially contributing to epileptogenesis.
Keywords: PSD-95; VGAT; VGlut1; VIP; VPAC1 receptor; gephyrin; hippocampus; theta-burst LTP.
Conflict of interest statement
The authors have no conflicts of interest in the publication of this paper.
Figures




References
MeSH terms
Grants and funding
- UIDB/04046/2020 (DOI: 10.54499/UIDB/04046/2020)/Fundação para a Ciência e Tecnologia
- UIDP/04046/2020 (DOI: 10.54499/UIDP/04046/2020)/Fundação para a Ciência e Tecnologia
- Norma Transitória - DL57/2016/CP1479/CT0044 to DCR (DOI: 10.54499/DL57/2016/CP1479/CT0044)/Fundação para a Ciência e Tecnologia
- FCT/POCTI (PTDC/SAUPUB/28311/2017) EPIRaft grant/Fundação para a Ciência e Tecnologia
- SFRH/BPD/81358/2011 to DCR/Fundação para a Ciência e Tecnologia
LinkOut - more resources
Full Text Sources
Research Materials

