Ethanol Extracts from Torreya grandis Seed Have Potential to Reduce Hyperuricemia in Mouse Models by Influencing Purine Metabolism
- PMID: 38540830
- PMCID: PMC10968862
- DOI: 10.3390/foods13060840
Ethanol Extracts from Torreya grandis Seed Have Potential to Reduce Hyperuricemia in Mouse Models by Influencing Purine Metabolism
Abstract
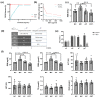
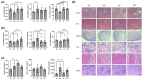
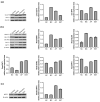
The purpose of this study was to evaluate the efficacy of ethanol extracts from Torreya grandis seed (EST) as a functional food in hyperuricemia mice. We investigated EST by analyzing its chemical composition. Using a mouse model of hyperuricemia induced by potassium oxonate (PO), we evaluated the effects of EST on uric acid (UA) production, inflammation-related cytokines, and gut microbiota diversity. The primary constituents of EST consist of various flavonoids and phenolic compounds known for their antioxidant and anti-inflammatory properties in vitro. Notably, our findings demonstrate that EST significantly reduced UA levels in hyperuricemia mice by 71.9%, which is comparable to the effects observed with xanthine treatment. Moreover, EST exhibited an inhibitory effect on xanthine oxidase activity in mouse liver, with an IC50 value of 20.90 μg/mL (36%). EST also provided protective effects to the mouse kidneys by modulating oxidative stress and inflammation in damaged tissues, while also enhancing UA excretion. Finally, EST influenced the composition of the intestinal microbiota, increasing the relative abundance of beneficial bacteria such as Akkermansia muciniphila, Corynebacterium parvum, Enterorhabdus, Muribaculaceae, Marvinbryantia, and Blautia. In summary, our research unveils additional functions of Torreya grandis and offers new insights into the future of managing hyperuricemia.
Keywords: ROS; Torreya grandis; functional food ingredients; gut microbiota; uric acid.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures


References
-
- Feng S., Wu S., Xie F., Yang C.S., Shao P. Natural compounds lower uric acid levels and hyperuricemia: Molecular mechanisms and prospective. Trends Food Sci. Technol. 2022;123:87–102. doi: 10.1016/j.tifs.2022.03.002. - DOI
-
- Otaki Y., Konta T., Ichikawa K., Fujimoto S., Iseki K., Moriyama T., Yamagata K., Tsuruya K., Narita I., Kondo M., et al. Possible burden of hyperuricaemia on mortality in a community-based population: A large-scale cohort study. Sci. Rep. 2021;11:8999. doi: 10.1038/s41598-021-88631-8. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

