The Impact on Survival of Neoadjuvant Treatment Interruptions in Locally Advanced Rectal Cancer Patients
- PMID: 38541008
- PMCID: PMC10971105
- DOI: 10.3390/jpm14030266
The Impact on Survival of Neoadjuvant Treatment Interruptions in Locally Advanced Rectal Cancer Patients
Abstract
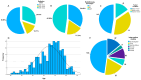
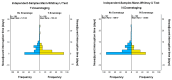
The standard oncologic treatment of locally advanced rectal cancer is long-course radio-chemotherapy followed by surgery and adjuvant chemotherapy. This can result in a lengthy total treatment duration, sometimes up to one year from the diagnosis. Interruptions to neoadjuvant treatment can occur for a variety of reasons, forced or unforced. The main purpose of this study is to analyze the survival data of locally advanced rectal cancer patients who received neoadjuvant treatment and to find a cut-off point showing exactly how many days of interruption of neoadjuvant treatment the risk of death or disease relapse increases. We conducted a retrospective study on 299 patients with locally advanced rectal cancer using survival analysis (Kaplan-Meier curve and Cox regression) to determine survival probabilities for overall survival, local control, and disease-free survival. Patients with 0 to 3 days of neoadjuvant therapy interruption had a higher overall survival probability compared to patients with 4 or more days (90.2% compared to 57.9%, p-value < 0.001), hazard ratio 5.89 (p < 0.001). Local control and disease-free survival had a higher probability in patients with 0-2 days of interruption compared to people with 3 or more days (94% vs. 75.4%, and 82.2% vs. 50.5%, respectively, both p-values < 0.001). Patients with tumoral or nodal downstaging experienced fewer days of interruption than patients with no downstage. These findings reinforce the need for radiation oncologists to be well-organized when starting neoadjuvant treatment for rectal cancer, in order to anticipate and prevent potential treatment interruptions and achieve the best therapeutic results.
Keywords: downstage; locally advanced rectal cancer; neoadjuvant radiotherapy; total neoadjuvant treatment; treatment interruptions.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Hashiguchi Y., Muro K., Saito Y., Ito Y., Ajioka Y., Hamaguchi T., Hasegawa K., Hotta K., Ishida H., Ishiguro M., et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2020;25:1–42. doi: 10.1007/s10147-019-01485-z. - DOI - PMC - PubMed
-
- Benson A.B., Venook A.P., Al-Hawary M.M., Azad N., Chen Y.J., Ciombor K.K., Cohen S., Cooper H.S., Deming D., Farkas L. NCCN Guidelines Version 4.2022 Rectal Cancer Continue NCCN Guidelines Panel Disclosures. 2023. [(accessed on 26 March 2023)]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf.
-
- Liscu H.-D., Liscu B.-R., Mitre R., Anghel I.-V., Antone-Iordache I.-L., Balan A., Coniac S., Miron A.-I., Halcu G. The Conditioning of Adjuvant Chemotherapy for Stage II and III Rectal Cancer Determined by Postoperative Pathological Characteristics in Romania. Medicina. 2023;59:1224. doi: 10.3390/medicina59071224. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

