Bimekizumab: Short-Term Effectiveness and Safety in Real Clinical Practice in Andalucia, Spain
- PMID: 38541607
- PMCID: PMC10971645
- DOI: 10.3390/life14030281
Bimekizumab: Short-Term Effectiveness and Safety in Real Clinical Practice in Andalucia, Spain
Abstract
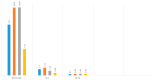
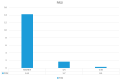
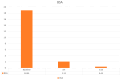
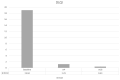
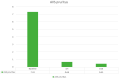
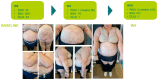
Introduction: Psoriasis, a chronic inflammatory skin disease, affects 2-10% of the population globally. Bimekizumab (BMK), a monoclonal antibody targeting IL-17, is a dual inhibitor of IL17 A and F that has shown efficacy in treating moderate to severe plaque psoriasis. This real-world evidence (RWE) study aims to assess BMK's efficiency and safety in naïve and refractory patients. Material and methods: A retrospective analysis of a multicenter observational study included 22 patients treated with BMK from April 2023 to February 2023 in five Andalusian hospitals. Ethical approval was obtained, and patients provided informed consent. Assessment criteria encompassed Psoriasis Area and Severity Index (PASI), body surface area (BSA), VAS pruritus, Dermatology Life Quality Index (DLQI), and minimum disease activity (MDA) at 0, 4, 12, and 24 weeks. Results: Patients, predominantly with plaque psoriasis, exhibited significant improvements in PASI (baseline 15.7 to 0.4 at week 16), BSA (baseline 20.7 to 0.43 at week 16), DLQI (baseline 17.93 to 0.43 at week 16), and pruritus (baseline 7.12 to 0.4 at week 16). At week 16, 95.4% achieved MDA. No safety concerns or treatment discontinuations were reported. Discussion: This RWE study aligns with pivotal clinical trials, confirming BMK's efficacy and safety. Notably, BMK demonstrated rapid and sustained psoriasis clearance, even in challenging areas. The study's limitations include a small sample size, suggesting the need for further exploration of patient-reported outcomes. Conclusion: Bimekizumab exhibited optimal efficacy and safety profiles in treating moderate to severe plaque psoriasis in a real-world setting. Rapid response, sustained clearance, and favorable safety outcomes contribute to improved patient experiences. Future research could delve into patient-reported outcomes and expand sample sizes to enhance the understanding of BMK's real-world effectiveness.
Keywords: bimekizumab; biological therapy; psoriasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Effectiveness and safety of bimekizumab for the treatment of plaque psoriasis: a real-life multicenter study-IL PSO (Italian landscape psoriasis).Front Med (Lausanne). 2023 Aug 8;10:1243843. doi: 10.3389/fmed.2023.1243843. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37614958 Free PMC article.
-
Fast Clinical Response of Bimekizumab in Nail Psoriasis: A Retrospective Multicenter 36-Week Real-Life Study.Pharmaceuticals (Basel). 2024 Oct 16;17(10):1378. doi: 10.3390/ph17101378. Pharmaceuticals (Basel). 2024. PMID: 39459016 Free PMC article.
-
Gender Influence on Bimekizumab Response in Patients with Psoriasis: Results of a Real-World Multicenter Retrospective Study-IL PSO (Italian Landscape PSOriasis).Dermatol Ther (Heidelb). 2025 Jul;15(7):1797-1811. doi: 10.1007/s13555-025-01435-w. Epub 2025 May 13. Dermatol Ther (Heidelb). 2025. PMID: 40358828 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2023 Jul 12;7(7):CD011535. doi: 10.1002/14651858.CD011535.pub6. Cochrane Database Syst Rev. 2023. PMID: 37436070 Free PMC article. Review.
-
Bimekizumab in the Treatment of Plaque Psoriasis: Focus on Patient Selection and Perspectives.Patient Prefer Adherence. 2023 Jun 30;17:1541-1549. doi: 10.2147/PPA.S350760. eCollection 2023. Patient Prefer Adherence. 2023. PMID: 37408843 Free PMC article. Review.
Cited by
-
Comparative Real-World Analysis of Baseline Demographic Characteristics and Comorbidities in Atopic Dermatitis Patients Initiating Biologics Versus JAK Inhibitors.J Clin Med. 2025 Feb 15;14(4):1291. doi: 10.3390/jcm14041291. J Clin Med. 2025. PMID: 40004820 Free PMC article.
References
-
- Puig L., Ruiz de Morales J.G., Dauden E., Andreu J.L., Cervera R., Adán A., Marsal S., Escobar C., Hinojosa J., Palau J., et al. La prevalencia de diez enfermedades inflamatorias inmunomediadas (IMID) en España. Rev. Esp. Salud Publica. 2019;93:e201903013. - PubMed
-
- European Public Assessment Report (EPAR) Bimzelx® (bimekizumab) [(accessed on 12 December 2021)]. Available online: https://www.ema.europa.eu/en/documents/assessment-report/bimzelx-epar-pu....
LinkOut - more resources
Full Text Sources
Miscellaneous

