Anticancer Potential of Pyridoxine-Based Doxorubicin Derivatives: An In Vitro Study
- PMID: 38541608
- PMCID: PMC10970924
- DOI: 10.3390/life14030282
Anticancer Potential of Pyridoxine-Based Doxorubicin Derivatives: An In Vitro Study
Abstract
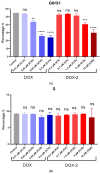
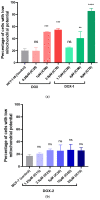
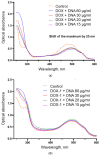
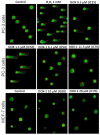
Doxorubicin (DOX) is a prevalent anticancer agent; however, it is unfortunately characterized by high cardiotoxicity, myelosuppression, and multiple other side effects. To overcome DOX limitations, two novel pyridoxine-derived doxorubicin derivatives were synthesized (DOX-1 and DOX-2). In the present study, their antitumor activity and mechanism of action were investigated. Of these two compounds, DOX-2, in which the pyridoxine fragment is attached to the doxorubicin molecule via a C3 linker, revealed higher selectivity against specific cancer cell types compared to doxorubicin and a promising safety profile for conditionally normal cells. However, the compound with a C1 linker (DOX-1) was not characterized by selectivity of antitumor action. It was revealed that DOX-2 obstructs cell cycle progression, induces apoptosis via the mitochondrial pathway without the development of necrosis, and showcases antioxidant capabilities, underlining its cell-regulatory roles. In contrast to doxorubicin's DNA-centric mechanism, DOX-2 does not interact with nuclear DNA. Given these findings, DOX-2 presents a new promising direction in cancer therapeutics, which is deserving of further in vivo exploration.
Keywords: DNA intercalation; anticancer agents; antioxidants; apoptosis induction; cell-cycle arrest; doxorubicin; doxorubicin derivatives; pyridoxine; vitamin B6.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








Similar articles
-
Algerian Propolis Potentiates Doxorubicin Mediated Anticancer Effect Against Human Pancreatic PANC-1 Cancer Cell Line through Cell Cycle Arrest, Apoptosis Induction and P-Glycoprotein Inhibition.Anticancer Agents Med Chem. 2018;18(3):375-387. doi: 10.2174/1871520618666180110143239. Anticancer Agents Med Chem. 2018. PMID: 29318976
-
Liposomes co-encapsulating doxorubicin and glucoevatromonoside derivative induce synergic cytotoxic response against breast cancer cell lines.Biomed Pharmacother. 2021 Apr;136:111123. doi: 10.1016/j.biopha.2020.111123. Epub 2021 Jan 22. Biomed Pharmacother. 2021. PMID: 33486211
-
Synergistic breast cancer suppression efficacy of doxorubicin by combination with glycyrrhetinic acid as an angiogenesis inhibitor.Phytomedicine. 2021 Jan;81:153408. doi: 10.1016/j.phymed.2020.153408. Epub 2020 Nov 4. Phytomedicine. 2021. PMID: 33234363
-
Molecular insights into the pathophysiology of doxorubicin-induced cardiotoxicity: a graphical representation.Arch Toxicol. 2022 Jun;96(6):1541-1550. doi: 10.1007/s00204-022-03262-w. Epub 2022 Mar 25. Arch Toxicol. 2022. PMID: 35333943 Free PMC article. Review.
-
The Protective Role of Phenolic Compounds Against Doxorubicin-induced Cardiotoxicity: A Comprehensive Review.Nutr Cancer. 2016 Aug-Sep;68(6):892-917. doi: 10.1080/01635581.2016.1187280. Epub 2016 Jun 24. Nutr Cancer. 2016. PMID: 27341037 Review.
Cited by
-
Doxorubicin-Induced Cardiotoxicity: A Comprehensive Update.J Cardiovasc Dev Dis. 2025 May 30;12(6):207. doi: 10.3390/jcdd12060207. J Cardiovasc Dev Dis. 2025. PMID: 40558642 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

