Primary Cutaneous CD4 Small/Medium T-Cell Lymphoproliferative Disorder Following COVID-19 Vaccination-What Do We Know about Lymphoproliferative Disorders and Cutaneous Lymphomas after COVID-19 Vaccination? A Report of an Atypical Case and a Review of the Literature
- PMID: 38541710
- PMCID: PMC10971988
- DOI: 10.3390/life14030386
Primary Cutaneous CD4 Small/Medium T-Cell Lymphoproliferative Disorder Following COVID-19 Vaccination-What Do We Know about Lymphoproliferative Disorders and Cutaneous Lymphomas after COVID-19 Vaccination? A Report of an Atypical Case and a Review of the Literature
Abstract
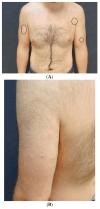
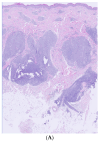
The association between Primary cutaneous CD4 small/medium T-cell lymphoproliferative disorder (PCSM-TCLPD) and COVID-19 immunization has been sparsely documented in the medical literature. Reviewing the literature, albeit infrequently, we can find cases of the recurrence and new onset of lymphoproliferative processes and cutaneous lymphomas following the COVID-19 vaccine. Many of the entities we encounter are classified as cutaneous lymphoproliferative disorders. The prevailing hypothesis suggests that the predominant cutaneous reactions to SARS-CoV-2 vaccines may stem from T-cell-mediated immune activation responses to vaccine components, notably messenger RNA (mRNA). Specifically, it is posited that the presence of cutaneous lymphoid infiltrates may be linked to immune system stimulation, supported by the absence, to date, of instances of primary cutaneous B-cell lymphoma following mRNA vaccination. Within this context, it is imperative to underscore that the etiological association between PCSM-TCLPD and COVID-19 vaccination should not discourage vaccination efforts. Instead, it underscores the necessity for continuous surveillance, in-depth investigation, and comprehensive follow-up studies to delineate the specific attributes and underlying mechanisms of such cutaneous manifestations post vaccination.
Keywords: CD4+ small/medium-sized T-cell lymphoproliferative disorder; COVID-19 vaccine; cutaneous lymphoma; cutaneous lymphoproliferative disorder.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Primary cutaneous CD4+ small/medium T-cell lymphoproliferative disorder in children: A case report and review of the literature.Pediatr Blood Cancer. 2022 Sep;69(9):e29862. doi: 10.1002/pbc.29862. Epub 2022 Jul 11. Pediatr Blood Cancer. 2022. PMID: 35730956 Review.
-
Primary cutaneous CD4-positive small/medium-sized pleomorphic T-cell lymphoproliferative disorder: Report of a case and review of the literature.J Cutan Pathol. 2017 Nov;44(11):944-947. doi: 10.1111/cup.13011. Epub 2017 Aug 30. J Cutan Pathol. 2017. PMID: 28749588 Review.
-
Clinical features and treatment outcomes for primary cutaneous CD4+ small/medium T-cell lymphoproliferative disorder: a retrospective cohort study from the Dana-Farber Cancer Institute and updated literature review.Leuk Lymphoma. 2022 Dec;63(12):2832-2846. doi: 10.1080/10428194.2022.2098287. Epub 2022 Jul 21. Leuk Lymphoma. 2022. PMID: 35862569 Review.
-
Cutaneous lymphoproliferative disorders after COVID-19 vaccination: clinical presentation, histopathology, and outcomes.Leuk Lymphoma. 2024 Jan;65(1):48-54. doi: 10.1080/10428194.2023.2270766. Epub 2024 Jan 10. Leuk Lymphoma. 2024. PMID: 37861685 Review.
-
Primary cutaneous marginal zone lymphoproliferative disorder following COVID-19 vaccination.J Cutan Pathol. 2024 Mar;51(3):193-197. doi: 10.1111/cup.14550. Epub 2023 Nov 28. J Cutan Pathol. 2024. PMID: 38018231
References
-
- McMahon D.E., Amerson E., Rosenbach M., Lipoff J.B., Moustafa D., Tyagi A., Desai S.R., French L.E., Lim H.W., Thiers B.H., et al. Cutaneous Reactions Reported after Moderna and Pfizer COVID-19 Vaccination: A Registry-Based Study of 414 Cases. J. Am. Acad. Dermatol. 2021;85:46–55. doi: 10.1016/j.jaad.2021.03.092. - DOI - PMC - PubMed
-
- Gambichler T., Boms S., Hessam S., Tischoff I., Tannapfel A., Lüttringhaus T., Beckman J., Stranzenbach R. Primary Cutaneous Anaplastic Large-cell Lymphoma with Marked Spontaneous Regression of Organ Manifestation after SARS-CoV-2 Vaccination. Br. J. Dermatol. 2021;185:1259–1262. doi: 10.1111/bjd.20630. - DOI - PMC - PubMed
-
- Avallone G., Maronese C.A., Conforti C., Fava P., Gargiulo L., Marzano A.V., Massone C., Mastorino L., Paradisi A., Pileri A., et al. Real-world Data on Primary Cutaneous Lymphoproliferative Disorders Following SARS-CoV-2 Vaccination: A Multicentre Experience from Tertiary Referral Hospitals. J. Eur. Acad. Dermatol. Venereol. 2022;37:e451–e455. doi: 10.1111/jdv.18806. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

